��Ŀ����
��0.1 mol��þ�������������100mL 2mol��L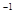 ��H2SO4��Һ�У�Ȼ���ٵμ�1mol��L
��H2SO4��Һ�У�Ȼ���ٵμ�1mol��L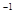 ��NaOH��Һ����ش𣺣�����Ҫд��������̣�
��NaOH��Һ����ش𣺣�����Ҫд��������̣�

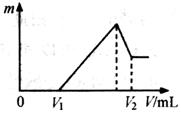
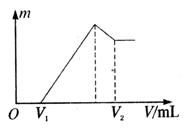
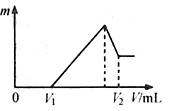
��1�����ڵμ�NaOH��Һ�Ĺ����г�������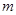 �����NaOH��Һ�����V�仯��ͼ��ʾ����
�����NaOH��Һ�����V�仯��ͼ��ʾ����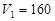 mLʱ���������ĩ��
mLʱ���������ĩ��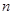 (Mg)= mol��
(Mg)= mol��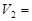 mL��
mL��
��2����Ҫ����100 mL 2mol��L ��H2SO4��Һ����Ҫ����������Ͳ���ձ��⣬���� ��
��H2SO4��Һ����Ҫ����������Ͳ���ձ��⣬���� ��
�����в�����ʹ�������ҺŨ��ƫС���ǣ� ��������ţ�
A������Ͳ��ȡһ�������98%��ŨH2SO4��ϡ�ͺ�δ����ȴ��ת������ƿ��
B��ϡ���������õ�С�ձ�δϴ��
C������ʱ����Һ��
D��������ˮϴ�Ӻ������ƿδ����
E������ҡ�Ⱥ��������Լ�ƿ�д���ʱ����������Һdz��ƿ��
���𰸡�
��1��0.06 440
��2���ٲ����� 100mL����ƿ����ͷ�ι� ��B
����������

��ϰ��ϵ�д�
�����Ŀ