��Ŀ����
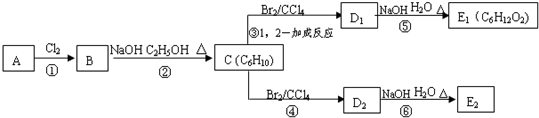
ij�������A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź����ױ���������ֻ��һ�����͵��⣮
��1��A�Ľṹ��ʽΪ
��2��A�е�̼ԭ���Ƿ���ͬһƽ�棿
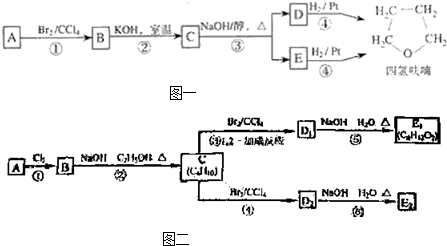
��3����ͼ�У�D1��D2��Ϊͬ���칹�壬E1��E2��Ϊͬ���칹�壮
��Ӧ�ڵĻ�ѧ����ʽΪ
C�Ļ�ѧ����Ϊ
E2�Ľṹ��ʽ��
�ܡ��ķ�Ӧ����������
��1��A�Ľṹ��ʽΪ
��CH3��2C=C��CH3��2
��CH3��2C=C��CH3��2
����2��A�е�̼ԭ���Ƿ���ͬһƽ�棿
��
��
����ǡ����ߡ����ǡ�������3����ͼ�У�D1��D2��Ϊͬ���칹�壬E1��E2��Ϊͬ���칹�壮

��Ӧ�ڵĻ�ѧ����ʽΪ
��CH3��2C��Cl��C��Cl����CH3��2+2NaOH
CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O
| �� |
| �� |
��CH3��2C��Cl��C��Cl����CH3��2+2NaOH
CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O
��| �� |
| �� |
C�Ļ�ѧ����Ϊ
2��3-����-1��3-����ϩ
2��3-����-1��3-����ϩ
��E2�Ľṹ��ʽ��
HOCH2C��CH3��=C��CH3��CH2OH
HOCH2C��CH3��=C��CH3��CH2OH
���ܡ��ķ�Ӧ����������
1��4-�ӳɷ�Ӧ��ȡ����Ӧ
1��4-�ӳɷ�Ӧ��ȡ����Ӧ
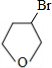
��������ij�������A������ͼ��������Է�������Ϊ84�������ΪCxHy����x���ֵΪ
=7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2BrCBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH���ݴ˽��
| 84 |
| 12 |
����⣺ij�������A������ͼ��������Է�������Ϊ84�������ΪCxHy����x���ֵΪ
=7��������ױ��������к���̼̼˫��������AΪϩ��������x=6��y=12�����A�Ļ�ѧʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵��⣬��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��A��������Ӧ����B��BΪ����CH3��2C��Cl��C��Cl����CH3��2��B���������ơ��Ҵ������¼��ȣ�������ȥ��Ӧ����C��CΪCH2=C��CH3��-C��CH3��=CH2��C���巢��1��2-�ӳ�����D1����D1ΪCH2Br-CBr��CH3��-C��CH3��=CH2��D1����������ˮ��Һ�з���ˮ�ⷴӦ����E1��E1ΪHOCH2C=CCH2OH��D1��D2��Ϊͬ���칹�壬�ʷ�Ӧ�ܷ���1��4-�ӳɣ�D2ΪCH2BrC��CH3��=C��CH3��CH2Br��D2����������ˮ��Һ�з���ˮ�ⷴӦ����E2��E2ΪHOCH2C��CH3��=C��CH3��CH2OH��
��1��������������֪��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2���ʴ�Ϊ����CH3��2C=C��CH3��2��
��2����CH3��2C=C��CH3��2�к���C=C˫����ƽ��ṹ��4������Cԭ�Ӵ���C=C˫����ƽ��ṹ�ڣ�����̼ԭ�Ӷ�����ͬһƽ�棬�ʴ�Ϊ���ǣ�
��3����Ӧ���ǣ�CH3��2C��Cl��C��Cl����CH3��2���������ƴ���Һ�����������·�����ȥ��Ӧ������CH2=C��CH3��-C��CH3��=CH2���÷�Ӧ�Ļ�ѧ����ʽΪ��
��CH3��2C��Cl��C��Cl����CH3��2+2NaOH
CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��
CΪCH2=C��CH3��-C��CH3��=CH2���Ļ�ѧ������2��3-����-1��3-����ϩ��
������������֪��E2�Ľṹ��ʽ��HOCH2C��CH3��=C��CH3��CH2OH��
��Ӧ����1��4-�ӳɷ�Ӧ����Ӧ����ȡ����Ӧ��
�ʴ�Ϊ����CH3��2C��Cl��C��Cl����CH3��2+2NaOH
CH2=C��CH3��-C��CH3��=CH2+2NaCl+2H2O��
2��3-����-1��3-����ϩ��HOCH2C��CH3��=C��CH3��CH2OH��1��4-�ӳɷ�Ӧ��ȡ����Ӧ��
| 84 |
| 12 |
��1��������������֪��A�Ľṹ��ʽΪ����CH3��2C=C��CH3��2���ʴ�Ϊ����CH3��2C=C��CH3��2��
��2����CH3��2C=C��CH3��2�к���C=C˫����ƽ��ṹ��4������Cԭ�Ӵ���C=C˫����ƽ��ṹ�ڣ�����̼ԭ�Ӷ�����ͬһƽ�棬�ʴ�Ϊ���ǣ�
��3����Ӧ���ǣ�CH3��2C��Cl��C��Cl����CH3��2���������ƴ���Һ�����������·�����ȥ��Ӧ������CH2=C��CH3��-C��CH3��=CH2���÷�Ӧ�Ļ�ѧ����ʽΪ��
��CH3��2C��Cl��C��Cl����CH3��2+2NaOH
| �� |
| �� |
CΪCH2=C��CH3��-C��CH3��=CH2���Ļ�ѧ������2��3-����-1��3-����ϩ��
������������֪��E2�Ľṹ��ʽ��HOCH2C��CH3��=C��CH3��CH2OH��
��Ӧ����1��4-�ӳɷ�Ӧ����Ӧ����ȡ����Ӧ��
�ʴ�Ϊ����CH3��2C��Cl��C��Cl����CH3��2+2NaOH
| �� |
| �� |
2��3-����-1��3-����ϩ��HOCH2C��CH3��=C��CH3��CH2OH��1��4-�ӳɷ�Ӧ��ȡ����Ӧ��
���������⿼���л����ƶϣ��漰±������ϩ�����ȵ������Լ�����ʽ����⡢ͬ���칹�塢�л���ѧ��Ӧ���ͺͷ���ʽ����д�ȣ���Ŀ�ۺ��Խϴ�ע���ϩ���ļӳɷ�Ӧ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
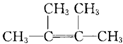

 +2NaOH
+2NaOH +2NaCl+2H2O
+2NaCl+2H2O
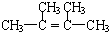




 +NaBr
+NaBr