��Ŀ����
�ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ����ͼ������ͼ���������գ�
��1���ڵ������У����Դ���������ĵ缫���������Ļ�ѧ��Ӧ����ʽΪ________�����Դ���������ĵ缫��������Һ��pH________����䡢���ߡ��½�����

��2����ҵʳ�κ�Ca2+��Mg2+�����ʣ����ƹ����з�����Ӧ�����ӷ���ʽΪ________��
��3�����������![]() �����ϸߣ��������ӱ��Լ���ȥ
�����ϸߣ��������ӱ��Լ���ȥ![]() ���ñ��Լ�������________��
���ñ��Լ�������________��
A��Ba(OH)2���������������������������� B��Ba(NO3)2���������������������������� C��BaCl2
��4��Ϊ��Ч��ȥCa2+��Mg2+��![]() �������Լ��ĺ���˳��Ϊ________��
�������Լ��ĺ���˳��Ϊ________��
A���ȼ�NaOH�����Na2CO3���ټӱ��Լ�
B���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
C���ȼӱ��Լ������NaOH���ټ�Na2CO3
��5�����ι���������NaOH��NaCl���ܽ���ϵIJ���ͨ��________����ȴ��_________����������ƣ���ȥNaCl��
��6���ڸ�Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Һ��Ӧ��
������Ĥ������ʳ��ˮ��Cl2��NaOH��Һ��ֽӴ����������NaClO��H2����Ӧ�Ļ�ѧ����ʽΪ________��
������
| ��1��2Cl--2e�TCl2 ���� ��2��Ca2++CO32-�TCaCO3�� Mg2++2OH-�TMg(OH)2��
��3��AC ��4��BC ��5������ ���� ��6��NaCl+H2O ��2����Mg(OH)2�ܽ�ȱ�MgCO3С����Mg2+������Mg2++2OH-�TMg(OH)2���� ��3����Ba(NO3)2����NO3-���ʣ�����ѡ�á� ��4������ʱ������Ba2+��OH-���Ⱥ�֮�֣���Na2CO3һ��Ҫ��BaCl2֮����룬��CO32-Ҫ������ȥ�����Ba2+��
|

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�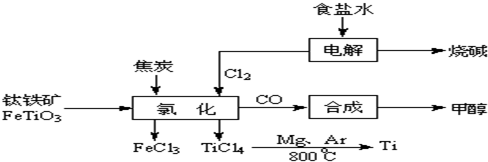
 2OH-+H2��+Cl2��
2OH-+H2��+Cl2�� 2MgCl2��s��+Ti����Ar�����н��е������ǣ�
2MgCl2��s��+Ti����Ar�����н��е������ǣ�





 ____________
____________

 ��Ar������������________
��Ar������������________