��Ŀ����
 ��1��д��ͼ���е������������ĵ缫��Ӧʽ�õ��ص��ܷ�Ӧʽ��
��1��д��ͼ���е������������ĵ缫��Ӧʽ�õ��ص��ܷ�Ӧʽ��������
Cu2++2e-=Cu
Cu2++2e-=Cu
��������
4OH--4e-=O2��+2H2O
4OH--4e-=O2��+2H2O
�������ܷ�Ӧʽ��
2CuSO4+2H2O
2Cu+O2��+2H2SO4
| ||
2CuSO4+2H2O
2Cu+O2��+2H2SO4
��
| ||
��2��д��ͼ����ԭ������������ĵ缫��Ӧʽ��ԭ��ص��ܷ�Ӧʽ��
������
2H++2e-=H2��
2H++2e-=H2��
��������
Zn-2e-=Zn2+
Zn-2e-=Zn2+
��ԭ����ܷ�Ӧʽ��
Zn+2HCl=ZnCl2+H2��
Zn+2HCl=ZnCl2+H2��
����������1�����Ե缫���缫���ϣ��������ͭ��Һʱ�����������������ӷŵ磬������ͭ���ӷŵ磬�ݴ�д���缫��Ӧʽ����ط�Ӧʽ��
��2��ͭ��п��ϡ���ṹ�ɵ�ԭ����У��ϻ��õĽ���п��������ͭ��������������пʧ���ӷ���������Ӧ�������������ӵõ��ӷ�����ԭ��Ӧ���ݴ�д���缫��Ӧʽ����ط�Ӧʽ��
��2��ͭ��п��ϡ���ṹ�ɵ�ԭ����У��ϻ��õĽ���п��������ͭ��������������пʧ���ӷ���������Ӧ�������������ӵõ��ӷ�����ԭ��Ӧ���ݴ�д���缫��Ӧʽ����ط�Ӧʽ��
����⣺��1�����Ե缫���缫���ϣ��������ͭ��Һʱ��������ͭ���ӷŵ磬�缫��ӦʽΪ��Cu2++2e-=Cu�����������������ӷŵ磬�缫��ӦʽΪ��4OH--4e-=O2��+2H2O����ط�ӦʽΪ��2CuSO4+2H2O
2Cu+O2��+2H2SO4��
�ʴ�Ϊ��Cu2++2e-=Cu��4OH--4e-=O2��+2H2O��2CuSO4+2H2O
2Cu+O2��+2H2SO4��
��2��ͭ��п��ϡ���ṹ�ɵ�ԭ����У��ϻ��õĽ���п��������ͭ�������������������ӵõ��������������缫��ӦʽΪ��2H++2e-=H2����������пʧ��������п���ӣ��缫��ӦʽΪZn-2e-=Zn2+����ط�ӦʽΪZn+2HCl=ZnCl2+H2����
�ʴ�Ϊ��2H++2e-=H2����Zn-2e-=Zn2+��Zn+2HCl=ZnCl2+H2����
| ||
�ʴ�Ϊ��Cu2++2e-=Cu��4OH--4e-=O2��+2H2O��2CuSO4+2H2O
| ||
��2��ͭ��п��ϡ���ṹ�ɵ�ԭ����У��ϻ��õĽ���п��������ͭ�������������������ӵõ��������������缫��ӦʽΪ��2H++2e-=H2����������пʧ��������п���ӣ��缫��ӦʽΪZn-2e-=Zn2+����ط�ӦʽΪZn+2HCl=ZnCl2+H2����
�ʴ�Ϊ��2H++2e-=H2����Zn-2e-=Zn2+��Zn+2HCl=ZnCl2+H2����
���������⿼��ԭ��غ͵���ԭ������ȷ���ӷŵ�˳���ǽⱾ��ؼ����缫��Ӧʽ����д�Ǹ߿��ȵ㣬Ӧ�������գ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


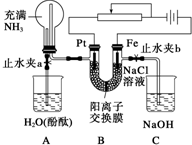 ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ������
ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ������
