��Ŀ����
ͬ���������ת���ķ�Ӧ���൱С����ת�����ʽ�������ʱ���ܲ���ȫ���ⶨ��Ӧ�Ⱥ����ѡ����ڿɸ��ݸ�˹����Ĺ۵㡰���ܻ�ѧ������һ����ɻ�ּ�����ɣ�����ܹ��̵���ЧӦ����ͬ�ġ�����֪��
P4(�̡�����)+5O2(��)=P4O10(��)+2983.2KJ���� ��
P(�̡�����)+![]()
![]() (��)=
(��)=![]()
![]() (��)+738.5KJ
��
(��)+738.5KJ
��
��д������ת��Ϊ�����Ȼ�ѧ����ʽ________��
�𰸣�
������
��ʾ��
������
P4(�̡�����)=4P(�̡�����)+29.2KJ
|
��ʾ��
�ݸ�˹�Ĺ۵㣬��������Ӧ����ʽ�����´����� ��
|

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��6�֣���գ�
��1���ڲⶨ����ͭ�ᾧˮ����ʵ������У������������ٽ��� �Ρ������ȵ� ����������
ʱ��ֹͣ���ȣ������������� ����ȴ���� ʱ�����Ϊ�ᾧˮ�Ѿ���ȫʧȥ��ʵ�ʲ����У���Щ������ʹʵ����ƫ��ƫ�͡����в�����ʹ�ⶨ���ƫ�ߵ��� ������ĸ��
| A�������¶ȹ��߶�ʹ����ͭ���ַֽ� | B��������ˮϴ��û�к�� |
| C�����Ⱥ���ڿ�������ȴ | D����ĩδ��ȫ���ֹͣ���� |
P(s������)��5/4O2(g)��1/4P4O10(s)����H����738.5kJ��mol��1
�ɴ˿�֪�����ȶ��ԱȺ��ףߣߣߣߣߡ�(��д��ǿ��������)
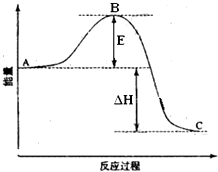 ��ͬ���������ת���ķ�Ӧ���൱�ٶ���ת�����ʽ�������ʱ���ܲ���ȫ���ⶨ��Ӧ�Ⱥ����ѣ����ڿɸ��ݸ�˹���ɽ��м��㣮
��ͬ���������ת���ķ�Ӧ���൱�ٶ���ת�����ʽ�������ʱ���ܲ���ȫ���ⶨ��Ӧ�Ⱥ����ѣ����ڿɸ��ݸ�˹���ɽ��м��㣮