��Ŀ����
CuSO4?5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�á�CuSO4?5H2O��ʵ�����Ʒ����£�
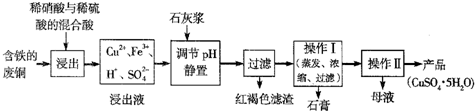
��ʵ�����ú�������(FeO��Fe2O3)�ķ�CuO�Ʊ��������徭�����й���(Fe3����pH��5ʱ����ȫ����)
�ش��������⣺
����98%��Ũ���������ܽ����õ�4.5 mol?L��1��ϡ���ᣬֻ�� �ֲ�������
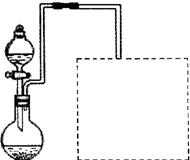
�Ʋ����Ŀ���ǽ���Һ���е�Fe2�����ת����Fe3����ʵ�������
������Ӧ�����ӷ���ʽ��
�����֤��Fe2���Ѿ����ת����
�Ȳ����֮���ʵ������� ����������������̨������Ȧ����
������װ�á�
��ʵ������ͭ���Ʊ���CuSO4?5H2O����ͼ���£�
�����������������գ�
�������ϣ�Ϊ���Ƶô�����CuSO4��5H2O���壬��Ҫ����ϡ���ᡢϡ�����������ʵ���֮��Ϊ ��
������Ӧ�����ӷ���ʽΪ ��
��ʵ���Ƶõĵ��������л��Ǻ���һЩ���ʣ�ͨ������ ���ᴿ��
��ʵ�����������ж�����ϡ�����Ũ�ȿ���Ҫ��Ƚϸߣ�ͨ���ñ�����������Һ���ζ����ζ����������÷�̪��ָʾ�����յ�������
��
����ͼ�л����ζ���������Һ��pH�����μ�����������Һ����ı仯������ͼ��Ҫ���A�㣩��
������ʹ�õı�����������Һ�Ѿ�ͨ�������ʵı궨������������ͨ���������궨��Һ�Ļ������� ��
| A������ | B������ | C�������� | D������ |
��5 �Ƽ�������H2O2 2 Fe2����H2O2��2 H����2 Fe3����2H2O
��ȡ��Һ������������(H��)KMnO4��Һ������Һ����ɫ����Fe2���ѳ��ת��
������Ũ������ȴ�ᾧ �������������ƾ��ơ�
��3��2��3Cu��8 H����2NO3����3Cu2����2NO����4H2O�� ���ؽᾧ
�Ǽ������һ�α�Һ����Һ��dz��ɫ����30s�ڲ���ɫ��
��BC��
����

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�| A�����ö����ЧӦ����Fe��OH��3�����FeCl3��Һ | B����Ʒ����Һ�����ֶ�����̼�Ͷ������� | C����CuSO4?5H2O����ƾ��к��е�ˮ | D����ɫ��ӦΪ��ɫ��ij��Һ��һ������Na+ |