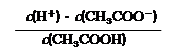��Ŀ����
��10�֣�
�������ճ�����������ĵ�ζ������Ҫ�Ļ���ԭ�ϣ����������䳣�����Ρ�
(��֪��25�棬����ĵ���ƽ�ⳣ��Ka(CH3COOH)��1��10��5���� ��ش�
�� д����������ˮ�з���ˮ�ⷴӦ�����ӷ���ʽ�� ��
�� ��CH3COONa��Һ������Ũ���ɴ�С��˳��Ϊ
���á�c(Bn��)����ʾ��Ӧ����Ũ�ȣ���
�� 25��ʱ������ĵ���ƽ�ⳣ������ʽKa�� ��0.10mol/L�Ĵ�����Һ��pH���� ����ʾ������ĵ��볣����С��ƽ��ʱ��c(CH3COOH)�ɽ�����Ϊ�Ե���0.10mol/L������
�� ���ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��CH3COONa��CH3COOH��Һ�������Ϻ���Һ��PH<7��ע�����ǰ����Һ����仯���Բ��ƣ������Һ�е����й�ϵʽ��ȷ���� ��
A��2c(Na��) ��c(CH3COO��)��c(CH3COOH)
B��c(Na��)��c(H��)��c(CH3COO��)��c(OH��)
C��c(CH3COO��)��c(CH3COOH)��0.1mol/L
D��c(Na��)>c(CH3COO��)> c(H��)> c(OH��)
��1��CH3COO-+ H2OCH3COOH+OH-
��2��C (Na+)��C (CH3COO-)��C (OH-) ��C( H+)
��3��Ka��[CH3COO-][ H+]/[ CH3COOH] ��3
��4��ABC
����:

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д��������ճ�����������ĵ�ζ������Ҫ�Ļ���ԭ�ϣ����������䳣�����Ρ�(��֪��25�棬Ka(CH3COOH)��1.69��10��5���� ��ش�
�� д����������ˮ�з���ˮ�ⷴӦ�����ӷ���ʽ�� ��
�� ��CH3COONa��Һ������Ũ���ɴ�С��˳��Ϊ
���á�c(Bn��)����ʾ��Ӧ����Ũ�ȣ���
�� 25��ʱ������ĵ���ƽ�ⳣ������ʽKa�� ��0.10mol/L�Ĵ�����Һ��pHԼΪ ����ʾ������ĵ��볣����С��ƽ��ʱ��c(CH3COOH)
�ɽ�����Ϊ�Ե���0.10mol/L�� ��֪��lg1.3��0.114����
�� ���ڴ�����Һ�ʹ�������Һ������˵����ȷ����
A��ϡ�ʹ�����Һ������ĵ���̶�����ϡ�ʹ�������Һ������Ƶ�ˮ��̶ȼ�С��
�����¶ȿ��Դٽ�������룬�������¶�������ƴ�����ˮ�⡣
C������ʹ����ƵĻ��Һ�У��������ƴ����Ƶ�ˮ�⡢������Ҳ���ƴ���ĵ��롣
D������ʹ����ƵĻ��Һ�У�����ٽ������Ƶ�ˮ�⡢������Ҳ�ٽ�����ĵ��롣
�� ���ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��CH3COONa��CH3COOH��Һ�������ϣ�ע�����ǰ����Һ����仯���Բ��ƣ������Һ�е����й�ϵʽ��ȷ���� ��
A��c(CH3COOH)��2c(H��)��c(CH3COO��)��2c(OH��)
B��c(Na��)��c(H��)��c(CH3COO��)��c(OH��)
C��c(CH3COO��)��c(CH3COOH)��0.1mol/L
�� ����ʱ��������3����Һ������pH��С����

�� ��֪�����ܹ���С�մ���Һ�������з�Ӧ��
CH3COOH��NaHCO3��CH3COONa��CO2����H2O ��
��pH��ֽ�ڳ����·ֱ�ⶨ0.10mol/L�Ĵ�������Һ��0.10mol/L��̼��������Һ��
��pH(CH3COONa) pH(NaHCO3)�������������������������