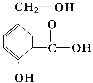
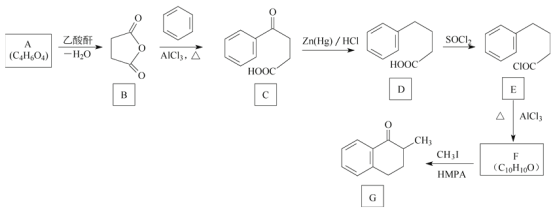
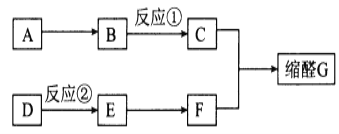
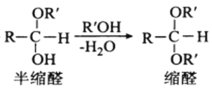��Ŀ����
����Ŀ�����ȵ���NaClO2��һ����Ҫ�ĺ����������������ǹ������ⷨ����NaClO2�Ĺ�������ͼ��
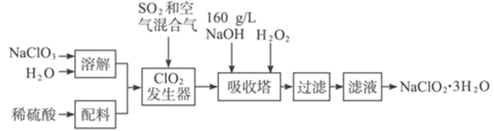
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
��ClO2�ķе�Ϊ283 K����ClO2�ֽⱬը������ϡ����������ϡ�ͷ�ֹ��ը�Էֽ�
(1)NaClO2 ������Ϊ_______________
(2)ClO2����������������Ӧ�Ļ�ѧ����ʽΪ_______________���������й�����������ÿ�����_______________��ѡ����ţ���
a����SO2������SO3����ǿ���ԣ� b��ϡ��ClO2�Է�ֹ��ը�� c����NaClO3������ClO2
(3)���չ��������û�ԭ���Ļ�ԭ��Ӧ���У�ԭ����_________����H2O2�⣬������ѡ��Ļ�ԭ����____________������ţ�
A��Na2O2 B��Na2S C��FeCl2 D��KMnO4
(4)����Һ�еõ�NaClO2��3H2O�־����ʵ�����������____________(ѡ�����)��
A������ B������ C������ D������ E����ȴ�ᾧ
(5)�����õ�NaClO2��3H2O�����ñ�ˮϴ�Ӻ�ɣ����ȷ���þ���ϴ�Ӹɾ���
________________________
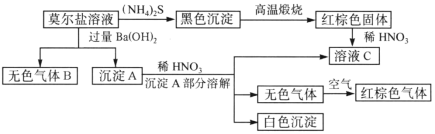
���𰸡��������� 2NaClO3��SO2��H2SO4��2ClO2��2NaHSO4��2NaClO3��SO2��2ClO2��Na2SO4 b ��ֹ����NaClO2��������ԭ��NaCl A BED ȡ���һ��ϴ��Һ�����������ữ��BaCl2��Һ�����Ƿ��г�������
��������
��ClO2��������NaClO3��H2SO4��SO2����Һ�з�����Ӧ����ClO2��NaHSO4����Ӧ������Һ��ͨ��������ɽ�ClO2�����������У�����NaOH��H2O2��������NaClO2��H2O2����������O2������ȴ�ᾧ���ؽᾧ������õ�NaClO23H2O���Դ˽����⡣
(1)NaClO2��ClԪ�ػ��ϼ�Ϊ+3�ۣ�����Ϊ�������ƣ�
(2)��ClO2��������NaClO3��H2SO4��SO2����Һ�з�����Ӧ����ClO2��NaHSO4����Ӧ�Ļ�ѧ����ʽΪ��2NaClO3��SO2��H2SO4��2ClO2��2NaHSO4����ClO2�ֽⱬը���������й��������������ϡ��ClO2�Է�ֹ��ը���ʺ���ѡ����b��
(3)�������з�����Ӧ�Ļ�ѧ����ʽΪ2ClO2+H2O2+2NaOH�T2NaClO2+2H2O+O2�������չ��������û�ԭ���Ļ�ԭ��Ӧ���У���Ϊ�˷�ֹ����NaClO2��������ԭ��NaCl��Ϊ�����������ʣ���ԭ��Ҳ����Ϊ�������ƣ��ʺ���ѡ����A��
(4)����Һ�еõ�NaClO2��3H2O�־����ʵ���������������Ũ������ȴ�ᾧ�����ˣ��ʺ���ѡ����BED��
(5)�����õ�NaClO2��3H2O�����ñ�ˮϴ�Ӻ�ɣ�������ϴ�Ӹɾ�����ϴ��Һ�в���SO42-����ȷ���þ���ϴ�Ӹɾ��IJ����ǣ�ȡ���һ��ϴ��Һ�����������ữ��BaCl2��Һ�����Ƿ��г������ɣ�������������˵������ϴ�Ӹɾ����������δϴ�Ӹɾ���