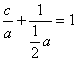��Ŀ����
��830Kʱ�����п��淴Ӧ�ﵽ��ƽ����ϵ����ijЩ�����ԣ�CO(g)��H2O(g)![]() CO2(g)��H2(g)
CO2(g)��H2(g)
(1)����ʼŨ�ȷֱ�Ϊc(CO)��3 mol��L-1��c(H2O)��0.75 mol��L-1ʱ���ﵽ��ƽ���CO��ת����Ϊ20����
(2)����ʼŨ�ȷֱ�Ϊc(CO)��3 mol��L-1��c(CO)��4.5 mol��L-1ʱ���ﵽ��ƽ���CO2�IJ���Ϊ60����
��ͨ�������ͼ���ش��������⣺
��830Kʱ��������ʼŨ�ȷֱ�Ϊc(CO)��a mol��L-1��c(H2O)��b mol��L-1Ͷ�ϣ��������Ϸ�Ӧ�����ƽ���c(H2)��c mol��L-1
(1)ѡ��ش��ڶ��ʵ���У�������b�������Сa����ﵽƽ���CO��ת����________��H2O��ת����_________��
A.����
B.����
C.����
D.��ȷ��
(2)��a��4��c��1.5ʱ��b��________��
(3)��a��26��a:c��________��
(4)a��b��c�ڸ�ƽ����ϵ�еĹ�ϵʽ��
������
| �����ѶȽϴ�Ҫ����������⣬�������������������ɵ�������
(1)[CO]����a(CO)��ֵ��С��a(H2O)��ֵ�� [CO]��С��a(CO)��ֵ����a(H2O)��ֵ��С�� ��ѡA��B�� (2)����ת����֮��Ϊ1���� ���b��2.4 (3) (4)
|
��ʾ��
| ������Ĺؼ���Ѱ�Ҹ���������ԡ�
��a(CO)��20����a(H2O)��80�� ��a(CO)��60����a(H2O)��40�� ������ɿ���������Ӧ���ת����֮��Ϊ1��
|

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�