��Ŀ����
�á��̡������ж�����˵���Ƿ���ȷ��
��1��һ��D2O����������������Ϊ8��
��2��HI��HBr��HCl��HF���ȶ���������ǿ��
��3��ͬ����Ԫ�ش��ϵ��£����ʵ��۵����͡�
��4��������Na������O2��Ӧ����Na2O�����¶���������Na2O�������ӿ졣
��5���������Ƕȿ����Ͽ���ѧ��Ҫ���ȣ��γɻ�ѧ��Ҫ���ȡ�һ����ѧ��Ӧ���ͷ���������������������ȡ���ڶ��ߵ���Դ�С��
��6����пƬ��ͭƬ�õ������ӣ���ƽ�в���ϡ�����У�����пƬ�Ǹ�����������Һ�е�H����Ǩ�ơ�
��7���ڶ��������������ķ�Ӧ�У��ʵ��������Ũ�ȣ�����߶��������ת���ʡ�
��8�����ȼ���û��ͬ���칹�壬֤��������Ӿ�����������ṹ��
��9���õ�ȼ��ͨ�����Ը��������Һ�еķ��������Լ���������ϩ��
��10�����������Ը��������Һ����ˮ������Ӧ��֤����������������ϩ�е�˫����
��1������2���̣�3������4������5������6������7���̣�8���̣�9���̣�10����

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
�����йز������ж���ȷ���ǣ�������
| A������һ�����ʵ���Ũ�ȵ���Һʱ������ʱ���ӿ̶��ᵼ��������ҺŨ��ƫ�� | B����������ƽ��ȡ25.20gNaCl | C����100mL����Ͳ��ȡ5.2mL������ | D����Ũ��������һ�����ʵ���Ũ�ȵ�ϡ���ᣬ��ȡŨ����ʱ������Ͳ�Ŀ̶��ᵼ��������ҺŨ��ƫ�� |
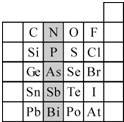 ��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
 ��仯ѧ���������о����̿���Ҫ�ɷ���MnO2���Ĺ����У�������Ũ�����ϼ��ȣ�������һ�ֻ���ɫ���̼�����ζ������--������ijѧ��ʹ����һԭ�������ͼ��ʾ��ʵ��װ�ã����������Ƶõ������볱ʪ����ʯ�ҷ�Ӧ��ȡ����Ư�ۣ�����һ�����ȷ�Ӧ�����ݴ˻ش��������⣺
��仯ѧ���������о����̿���Ҫ�ɷ���MnO2���Ĺ����У�������Ũ�����ϼ��ȣ�������һ�ֻ���ɫ���̼�����ζ������--������ijѧ��ʹ����һԭ�������ͼ��ʾ��ʵ��װ�ã����������Ƶõ������볱ʪ����ʯ�ҷ�Ӧ��ȡ����Ư�ۣ�����һ�����ȷ�Ӧ�����ݴ˻ش��������⣺