��Ŀ����
 ��������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ��������Ҫ�������м���Һ�����壩��
��������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ��������Ҫ�������м���Һ�����壩��
��ش��������⣺
��1�����������ﰱ����װ����______������ĸ����
��2�����������ռ��������������ռ�һ�����������װ����______������ĸ����
��3��������������Ӧʵ���У����������������ͷ�Ӧװ��֮���Գ�ȥ�������Ȼ�������������װ����______������ĸ����
��4����������ϩ����ˮ��Ӧ�ƶ��������ʵ��װ����______������ĸ����
��5������Cװ���������������ձ�������������Һ��Ӧ��ʵ�飬�����й��ƿ��������______��
��6��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ijͬѧ�������ͼ��ʾ��ʵ�飮

�ٿ�ͨ���۲�______���������ԱȽϵó����ۣ�
����ͬѧ�����CuSO4��ΪCuCl2��Ϊ��������������______������Ϊ���������θĽ���______�q
�⣺��1������Ϊ�������壬���ü��Ը������ʯ�ҹ���������ʯ��ֻ����װ��D��E�У��ʴ�Ϊ��D��E��
��2��һ����������������Ӧ��ֻ������ˮ���ռ���ֻ����B���ʴ�Ϊ��B��
��3����ȥ�������Ȼ�������������װ��ӦΪϴ��ƿ��A������ϴ��ƿ���ʴ�Ϊ��A��
��4��Aװ�ÿ�������ϩ����ˮ��Ӧ�ƶ��������ʵ��װ�ã���ϩ�ӳ����ܽ��룬ƿ��װ����ˮ���ʴ�Ϊ��A��
��5�������������弫������ˮ��Ҫ��ֹ������C����ƿ��ȫƿ�����ã��ʴ�Ϊ����ֹ������
��6���ٱȽϷ�Ӧ���ʵĴ�С��ͨ����������Ŀ������жϣ��ʴ�Ϊ������2H2O2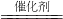 2H2O+O2����֪����������Ŀ������жϷ�Ӧ�Ŀ�����
2H2O+O2����֪����������Ŀ������жϷ�Ӧ�Ŀ�����
�ڱȽϲ�ͬ�����Ĵ�Ч����Ӧ�ų��������صĸ��ţ��ʴ�Ϊ������CuSO4��FeCl3�е������Ӳ�ͬ�������ų������ӵ����أ���FeCl3��ΪFe2��SO4��3��
��������1������Ϊ�������壬���ü��Ը������ʯ�ҹ������Դ�ѡ������������
��2��һ����������������Ӧ��ֻ������ˮ���ռ���
��3����ȥ�������Ȼ�������������װ��ӦΪϴ��ƿ��ֻ��A���ԣ�
��4����ϩ����ˮ��Ӧ�ƶ��������ʵ��Ϊ�����Һ��ķ�Ӧ��������������˫����Ƥ����
��5�������������弫������ˮ��Ҫ��ֹ������
��6���ٱȽϷ�Ӧ���ʵĴ�С��ͨ����������Ŀ������жϣ�
�ڱȽϲ�ͬ�����Ĵ�Ч����Ӧ�ų��������صĸ��ţ�
���������⿼�鳣����ѧ������ʹ�ã��漰����ľ������ռ�ʵ�鰲ȫ��ʵ��̽���ĵ����⣬��Ŀ�ѶȲ���ע��Aƿ������������ƿ��ϴ��ƿ����ȫƿ������ƿ�ȣ�
��2��һ����������������Ӧ��ֻ������ˮ���ռ���ֻ����B���ʴ�Ϊ��B��
��3����ȥ�������Ȼ�������������װ��ӦΪϴ��ƿ��A������ϴ��ƿ���ʴ�Ϊ��A��
��4��Aװ�ÿ�������ϩ����ˮ��Ӧ�ƶ��������ʵ��װ�ã���ϩ�ӳ����ܽ��룬ƿ��װ����ˮ���ʴ�Ϊ��A��
��5�������������弫������ˮ��Ҫ��ֹ������C����ƿ��ȫƿ�����ã��ʴ�Ϊ����ֹ������
��6���ٱȽϷ�Ӧ���ʵĴ�С��ͨ����������Ŀ������жϣ��ʴ�Ϊ������2H2O2
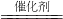 2H2O+O2����֪����������Ŀ������жϷ�Ӧ�Ŀ�����
2H2O+O2����֪����������Ŀ������жϷ�Ӧ�Ŀ������ڱȽϲ�ͬ�����Ĵ�Ч����Ӧ�ų��������صĸ��ţ��ʴ�Ϊ������CuSO4��FeCl3�е������Ӳ�ͬ�������ų������ӵ����أ���FeCl3��ΪFe2��SO4��3��
��������1������Ϊ�������壬���ü��Ը������ʯ�ҹ������Դ�ѡ������������
��2��һ����������������Ӧ��ֻ������ˮ���ռ���
��3����ȥ�������Ȼ�������������װ��ӦΪϴ��ƿ��ֻ��A���ԣ�
��4����ϩ����ˮ��Ӧ�ƶ��������ʵ��Ϊ�����Һ��ķ�Ӧ��������������˫����Ƥ����
��5�������������弫������ˮ��Ҫ��ֹ������
��6���ٱȽϷ�Ӧ���ʵĴ�С��ͨ����������Ŀ������жϣ�
�ڱȽϲ�ͬ�����Ĵ�Ч����Ӧ�ų��������صĸ��ţ�
���������⿼�鳣����ѧ������ʹ�ã��漰����ľ������ռ�ʵ�鰲ȫ��ʵ��̽���ĵ����⣬��Ŀ�ѶȲ���ע��Aƿ������������ƿ��ϴ��ƿ����ȫƿ������ƿ�ȣ�

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д�
�����Ŀ
 ��������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ��������Ҫ�������м���Һ�����壩��
��������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ��������Ҫ�������м���Һ�����壩��



 ��4��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ijͬѧ�������ͼ��ʾ��ʵ�顣
��4��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ijͬѧ�������ͼ��ʾ��ʵ�顣