��Ŀ����
Ŀǰ���������һ�����Ա�����Ʒ������Ҫ�ɷ���ƻ���ᡣƻ�����ڷ����ᴿ��Ļ�ѧ�������£�����Է�������������250����ȫȼ�պ�ֻ����CO2��H2O��������C������������35.82%��H����������4.48%����1 mol������������NaHCO3��Ӧ�ų�44.8 L CO2����������Na��Ӧ�ų�33.6 L H2(����������ڱ�״���²���)���۸÷����д������ֻ�ѧ������ͬ��̼ԭ�ӣ���ԭ��Ҳ�������ֲ�ͬ�Ļ�ѧ�����С�
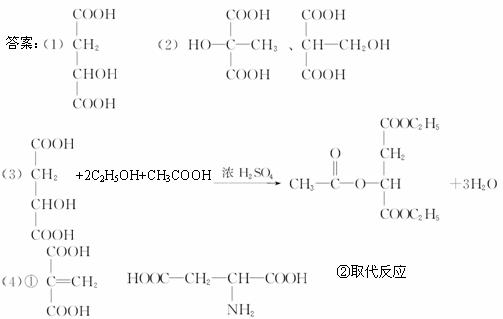
(1)д��ƻ����Ľṹ��ʽ��___________________________��
(2)��ƻ�����ͬ���칹���У����������٢�������������(д���ṹ��ʽ)��______________��
(3)д��ƻ����+�Ҵ�+������ŨH2SO4�����µĻ�ѧ����ʽ��___________________________��
(4)ƻ������Է�������ת����

��֪BΪ��Ԫ��״�ṹ��C��ʹ��ˮ��ɫ��E��������嵰���ʵİ�����֮һ�������ʽΪC4H7O4N��
��д����C������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ_________��E�Ľṹ��ʽ_________��
��D![]() E�ķ�Ӧ������_________��
E�ķ�Ӧ������_________��


 +2C2H5OH+CH3COOH
+2C2H5OH+CH3COOH

 ��ȡ����Ӧ
��ȡ����Ӧ
����:
��C��H��O�������������������ʽC4H6O5��1 mol������NaHCO3��Ӧ����2 mol CO2����֪��2����COOH����Na��Ӧ����1.5 mol H2��֪��3������H������2����COOH�⣬����һ����OH���־ݢ�����Ϣ��д���ṹ��ʽ�����(1)(2)(3)�������⡣��(4)�ṩ����Ϣ����ÿ����Ӧ����������д��������Ľṹ��ʽ��