��Ŀ����
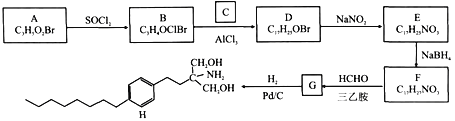
����Ŀ����A��CΪԭ�Ϻϳ����ƶ��Ӳ��֢ҩ��H��·�����£�
��֪��
��A����NaHCO3��Һ��Ӧ�ų�CO2����˴Ź���������ʾ������壬�������Ϊ2:2:1��
��NaBH4��ѡ���Ի�ԭȩ��ͪ��������ԭ��NO2��

�ش��������⣺
(1)A�Ļ�ѧ����Ϊ________��D�Ľṹ��ʽΪ_______��
(2)H�ķ���ʽΪ_______��E�й����ŵ�����Ϊ_______��
(3)B��D��E��F�ķ�Ӧ���ͷֱ�Ϊ_______��
(4)F��G�Ļ�ѧ����ʽΪ________��
(5)��C��Ϊͬ���칹����л���Ľṹ��ʽΪ_______(�˴Ź�������Ϊ����壬�������Ϊ6:3:1:1)��
(6)�����B��![]() Ϊԭ���Ʊ����п���������ҩ��
Ϊԭ���Ʊ����п���������ҩ�� �ĺϳ�·��__________��
�ĺϳ�·��__________��
���𰸡�3-����� ![]() C19H33NO2 �ʻ������� ȡ������ԭ
C19H33NO2 �ʻ������� ȡ������ԭ 


��������
A����ʽ��C3H5O2Br���ܹ���NaHCO3��Һ��Ӧ�ų�CO2��˵����������ᣬ�˴Ź���������ʾ������壬�������Ϊ2:2:1��A�ṹ��ʽΪBrCH2CH2COOH��A��SOCl2������Ϣ�۷�Ӧ����B��BrCH2CH2COCl������D�ķ���ʽ�����������H�Ľṹ��ʽ��֪C��![]() ��B��C
��B��C![]() ����������λ��ȡ����Ӧ����D�ṹ��ʽ��
����������λ��ȡ����Ӧ����D�ṹ��ʽ��![]() ��D��NaNO2����ȡ����Ӧ����E��
��D��NaNO2����ȡ����Ӧ����E��![]() ��E��NaBH4�����ʻ��Ļ�ԭ��Ӧ����F��
��E��NaBH4�����ʻ��Ļ�ԭ��Ӧ����F��![]() ��F��HCHO�����Ұ�����ʱ������Ϣ�ܷ�Ӧ����G��
��F��HCHO�����Ұ�����ʱ������Ϣ�ܷ�Ӧ����G�� ��G��H2������ԭ��Ӧ����H��
��G��H2������ԭ��Ӧ����H�� ���ݴ˽��
���ݴ˽��
��������������֪A��BrCH2CH2COOH��B��BrCH2CH2COCl��C��![]() ��D��
��D��![]() ��E��
��E��![]() ��F��
��F��![]() ��G��
��G�� ��H��
��H�� ��
��
(1)��������������֪A��BrCH2CH2COOH��A�Ļ�ѧ����Ϊ3-����D�Ľṹ��ʽΪ![]() ��
��
(2)����H�Ľṹ��ʽ ����֪H�ķ���ʽΪC19H33NO2��E�ṹ��ʽ��
����֪H�ķ���ʽΪC19H33NO2��E�ṹ��ʽ��![]() ��E�й����ŵ�����Ϊ�ʻ���������
��E�й����ŵ�����Ϊ�ʻ���������
(3) B��C![]() ����������λ��ȡ����Ӧ����D������B��D�ķ�Ӧ����Ϊȡ����Ӧ��E��NaBH4�����ʻ��Ļ�ԭ��Ӧ����F������E��F�ķ�Ӧ����Ϊ��ԭ��Ӧ��
����������λ��ȡ����Ӧ����D������B��D�ķ�Ӧ����Ϊȡ����Ӧ��E��NaBH4�����ʻ��Ļ�ԭ��Ӧ����F������E��F�ķ�Ӧ����Ϊ��ԭ��Ӧ��
(4)F��G�Ļ�ѧ����ʽΪ ��
��
(5)C��![]() ����C��Ϊͬ���칹�壬�˴Ź�������Ϊ����壬�������Ϊ6��3��1��1���л���Ľṹ��ʽΪ
����C��Ϊͬ���칹�壬�˴Ź�������Ϊ����壬�������Ϊ6��3��1��1���л���Ľṹ��ʽΪ ��
��
(6)B![]() ��
��![]() ����ȡ����Ӧ����
����ȡ����Ӧ���� ��
�� ��NaNO2����ȡ����Ӧ����
��NaNO2����ȡ����Ӧ���� ����������NaBH4�����ʻ��Ļ�ԭ��Ӧ����
����������NaBH4�����ʻ��Ļ�ԭ��Ӧ���� ��Ȼ����HCHO�����Ұ�����ʱ������Ϣ�ܷ�Ӧ����
��Ȼ����HCHO�����Ұ�����ʱ������Ϣ�ܷ�Ӧ���� ��Ȼ����H2��Cd/C�����·�����ԭ��Ӧ����
��Ȼ����H2��Cd/C�����·�����ԭ��Ӧ���� ���ʺϳ�·��Ϊ��
���ʺϳ�·��Ϊ�� ��
��

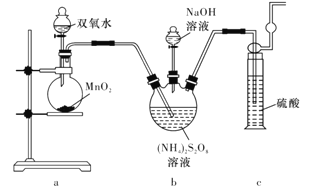
����Ŀ������ʵ������������۾���ȷ����
ʵ����������� | ���� | |
A | ȡ1 mL 20%��������Һ������3~5��ϡ���ᡣˮԡ����5 min��ȡ������Һ��������������Cu(OH)2�����ȣ���ש��ɫ�������� | ����û�з���ˮ�� |
B | ��װ����ˮ�ķ�Һ©���м����ѻ����ͣ���������ã��²�Ϊ��ɫ | �ѻ����Ϳ�����ȡ�� |
C | ��SO2ͨ����ɫʯ����Һ�У���Һ�ȱ�����ɫ | SO2�����������������Ư���� |
D | �ֱ���ʢ��KI3��Һ��a��b�Թ��еμӵ�����Һ��AgNO3��Һ��a����Һ������b�в�����ɫ���� | ��Һ�д��ڣ�I3�� |
A. AB. BC. CD. D