��Ŀ����
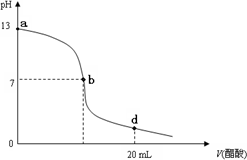
��20 mL����������Һ����μ���0. 2 mol/L������Һ���ζ���������ͼ��ʾ����˵������ȷ����
- A.������������Һ�����ʵ���Ũ��Ϊ0.1 mol/L ��
- B.��b�㣬c (Na+��=c(CH3COO-��
- C.��d�㣬��Һ����������Ũ���ɴ�С��˳��Ϊ
c (CH3COO-��>c (Na+)>c (H+��>c (OH-�� - D.����������Һ�������Һǡ����ȫ��Ӧ�ĵ�λ������b��d���ij��
D
��ͼ��ã�V�����ᣩ=0ʱ��PH=13��������������Һ��PH=13����C��OH-��=0.1 mol/L����A��ȷ��
��b��ʱ��PH=7����[H+]=[OH-]�������õ���غ㣺[Na+]+[H+]=[CH3COO-]+[OH-],��B��ȷ��
��d��ʱ��NaOH+CH3COOH=CH3COONa+H2O��Ӧ����Һ���Ϊ��Ũ�ȵ�CH3COOH��CH3COONa�Ļ���ǰ�߿��ǵ���ƽ�⣬���߿���ˮ��ƽ�⣩��PH��7������c (H+��>c (OH-������ͬ�����ڵ���غ㣺[Na+]+[H+]=[CH3COO-]+[OH-], ��[Na+]��[CH3COO-]����C��ȷ��
����������Һ�������Һǡ����ȫ��Ӧʱ������CH3COONa��CH3COONaˮ��ʼ��ԣ�PH>7����Ӧ��λ������a��b���ij�㣬��D����
��ͼ��ã�V�����ᣩ=0ʱ��PH=13��������������Һ��PH=13����C��OH-��=0.1 mol/L����A��ȷ��
��b��ʱ��PH=7����[H+]=[OH-]�������õ���غ㣺[Na+]+[H+]=[CH3COO-]+[OH-],��B��ȷ��
��d��ʱ��NaOH+CH3COOH=CH3COONa+H2O��Ӧ����Һ���Ϊ��Ũ�ȵ�CH3COOH��CH3COONa�Ļ���ǰ�߿��ǵ���ƽ�⣬���߿���ˮ��ƽ�⣩��PH��7������c (H+��>c (OH-������ͬ�����ڵ���غ㣺[Na+]+[H+]=[CH3COO-]+[OH-], ��[Na+]��[CH3COO-]����C��ȷ��
����������Һ�������Һǡ����ȫ��Ӧʱ������CH3COONa��CH3COONaˮ��ʼ��ԣ�PH>7����Ӧ��λ������a��b���ij�㣬��D����

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
|
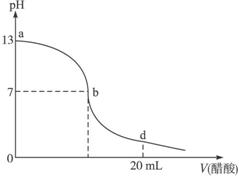
��20 mL����������Һ����μ���0.2 mol/L������Һ���ζ���������ͼ��ʾ����˵������ȷ����
| |
| [����] | |
A�� |
������������Һ�����ʵ���Ũ��Ϊ0.1 mol/L�� |
B�� |
��b�㣬c(Na+)��c(CH3COO��) |
C�� |
��d�㣬��Һ����������Ũ���ɴ�С��˳��Ϊc(CH3COO��)��c(Na+)��c(H+)��c(OH��) |
D�� |
����������Һ�������Һǡ����ȫ��Ӧ�ĵ�λ������b��d���ij�� |
��20 mL����������Һ����μ���0. 2 mol/L������Һ���ζ���������ͼ��ʾ����˵������ȷ���� �� �� 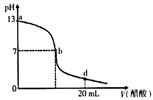
| A��������������Һ�����ʵ���Ũ��Ϊ0.1 mol/L �� |
| B����b�㣬c (Na+��=c(CH3COO-�� |
| C����d�㣬��Һ����������Ũ���ɴ�С��˳��Ϊ c (CH3COO-��>c (Na+)>c (H+��>c (OH-�� |
| D������������Һ�������Һǡ����ȫ��Ӧ�ĵ�λ������b��d���ij�� |