��Ŀ����
����Ŀ������A��B��C��D��E��F���ֶ���������Ԫ�أ�ԭ����������������֪A����̬�⻯������������������ˮ���ﷴӦ�õ�һ�����ӻ����B��һ�ֵ��ʾ���ɱ�����������ã�C����D3+�ĵ��Ӳ�ṹ��ͬ��E������������������������2����
��1��F��Ԫ�����ڱ��е�λ����__________________��
��2������Ԫ���γɵļ������У��뾶������____________(�����ӷ���)��
��3��������Ԫ���е�һ�ֻ�����ɵ����ʼ��Է������·�Ӧ��

����������Ư���ԣ����ˮ��Ӧ�����ӷ���ʽΪ__________________________��
�����ҵ�ˮ��Һ��ǿ������Һ�����ʱ�ΪB��һ�ֵ��ʣ�����к��еĻ�ѧ��������Ϊ________��D�ĵ������ҵ�ˮ��Һ��Ӧ�����ӷ���ʽΪ_________________��
������ΪD��E�γɵĶ�Ԫ��������ʱ������壬����ĽṹʽΪ___________��������Ϊ��ɫ�������仯ѧʽΪ______________��
���𰸡��������ڵڢ�A�� S2�� Cl2+H2O![]() H+ + Cl��+ HClO ���Ӽ� ���ۼ���Ǽ��Թ��ۼ� 2Al + 2OH�� + 2H2O =2AlO2��+ 3H2�� H��S��H Al(OH)3
H+ + Cl��+ HClO ���Ӽ� ���ۼ���Ǽ��Թ��ۼ� 2Al + 2OH�� + 2H2O =2AlO2��+ 3H2�� H��S��H Al(OH)3
��������
A��B��C��D��E��F���ֶ���������Ԫ�أ�ԭ����������������֪A����̬�⻯������������������ˮ���ﷴӦ�õ�һ�����ӻ����˵��AΪ��Ԫ�أ����ӻ�����Ϊ����泥�B��һ�ֵ��ʾ���ɱ�����������ã�Ϊ��Ԫ�أ�C����D3+�ĵ��Ӳ�ṹ��ͬ��˵��CΪ�ƣ�DΪ����E������������������������2����˵��Ϊ��Ԫ�ء���FΪ��Ԫ�ء�
(1)FΪ��Ԫ�أ��ڵ������ڵڢ�A�塣
(2)����Ԫ�صļ������У����Ӳ�����뾶��ͬ���Ӳ��������ӣ��˵����Խ�뾶ԽС�����뾶������ S2����
(3) �����ʼ�ˮ��Ӧ�����Һͱ���������Ư���ԣ�˵����������ˮ��Ӧ��������ʹ����ᣬ���ӷ���ʽΪ��Cl2+H2O![]() H+ + Cl��+ HClO��
H+ + Cl��+ HClO��
�����ҵ�ˮ��Һ��ǿ������Һ�����ʱ�ΪB��һ�ֵ��ʣ�˵�����ʼ�Ϊ�������ƣ�����������ˮ��Ӧ�����������ƺ��������������ƺ������Ӽ����ۼ���Ǽ��Թ��ۼ�����������������Һ��Ӧ�����ӷ���ʽΪ��2Al + 2OH�� + 2H2O =2AlO2��+ 3H2����
��������ˮ��Ӧ�����������������⣬����ĽṹʽΪH��S��H��������Ϊ Al(OH)3��

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�����Ŀ����֪��ѧ��Ӧ��:Fe(s)+CO2(g)![]() FeO(s)+CO(g),�仯ѧƽ�ⳣ��ΪK1;��ѧ��Ӧ��:Fe(s)+H2O(g)
FeO(s)+CO(g),�仯ѧƽ�ⳣ��ΪK1;��ѧ��Ӧ��:Fe(s)+H2O(g)![]() FeO(s)+H2(g),�仯ѧƽ�ⳣ��ΪK2,���¶�973 K��1173 K�������,K1��K2��ֵ�ֱ�����:
FeO(s)+H2(g),�仯ѧƽ�ⳣ��ΪK2,���¶�973 K��1173 K�������,K1��K2��ֵ�ֱ�����:
�¶� | K1 | K2 |
973 K | 1.47 | 2.38 |
1 173 K | 2.15 | 1.67 |
(1)ͨ�������е���ֵ�����ƶ�:��Ӧ����_______(��������������������)��Ӧ��
(2)���з�Ӧ��:CO2(g)+H2(g)![]() CO(g)+H2O(g),����д���÷�Ӧ��ƽ�ⳣ��K3�ı���ʽ:K3=______��
CO(g)+H2O(g),����д���÷�Ӧ��ƽ�ⳣ��K3�ı���ʽ:K3=______��
(3)���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵʽΪ__________,�ݴ˹�ϵʽ���ϱ�����,���ƶϳ���Ӧ����________(��������������������)��Ӧ��
(4)Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ�,�ɲ�ȡ�Ĵ�ʩ��______ ��_____ (��д��ĸ���)��
A.��С��Ӧ�������ݻ� B.����Ӧ�������ݻ�
C.�����¶� D.ʹ�ú��ʵĴ���
E.�跨��Сƽ����ϵ�е�CO��Ũ��
(5)ͼ�ס��ҷֱ��ʾ��Ӧ����t1ʱ�̴ﵽƽ��,��t2ʱ����ı�ij�������������仯�����:
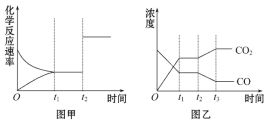
��ͼ����t2ʱ�̷����ı��������__________��
��ͼ����t2ʱ�̷����ı��������__________��