��Ŀ����
��1��Co��NH3��5BrSO4���γ������ܵ�������֪���������ķ���ʽ�ֱ�Ϊ[Co��NH3��5Br]SO4 ��[Co ��SO4�� ��NH3��5]Br���ڵ�һ����������Һ�м�BaCl2 ��Һʱ������
��2����AgNO3��Һ����μ���ϡ��ˮ���˹��̵�����Ϊ
�а�ɫ��������
�а�ɫ��������
��������ڵڶ�����������Һ�м���BaCl2��Һʱ������������
������
���������� AgNO3��Һʱ�������е���ɫ��������
�е���ɫ��������
������2����AgNO3��Һ����μ���ϡ��ˮ���˹��̵�����Ϊ
�����ɰ�ɫ�������ɫ��������ʧ��Һ�����
�����ɰ�ɫ�������ɫ��������ʧ��Һ�����
��д���ù��̵����ӷ���ʽΪAg++NH3?H2O�TAgOH��+NH4+��AgOH+2NH3?H2O�TAg��NH3��2++OH?+2H2O
Ag++NH3?H2O�TAgOH��+NH4+��AgOH+2NH3?H2O�TAg��NH3��2++OH?+2H2O
����������1��[Co��NH3��5Br]SO4 ����������Ӻͱ����ӷ�Ӧ�������ᱵ��ɫ������[Co ��SO4�� ��NH3��5]Br�в��������ƶ�����������ӣ����Բ��ܲ�����ɫ�����������Ӻ������ӷ�Ӧ���ɵ���ɫ������
��2���������Ͱ�ˮ��Ӧ���������������������������Ͱ�ˮ��Ӧ����������Һ��
��2���������Ͱ�ˮ��Ӧ���������������������������Ͱ�ˮ��Ӧ����������Һ��
����⣺��1����[Co��NH3��5Br]SO4��֪�����������Ϊ��������磬��ˮ��Һ����������ʽ���ڣ����Ի��뱵���ӽ�ϳɰ�ɫ������[Co��SO4����NH3��5]Br�����������Ϊ�Ƚ磬��ˮ��Һ�ﲻ�������Ӵ��ڣ����Լ���BaCl2��Һʱ������������������������Ϊ��������磬�ܺ������ӷ�Ӧ���ɵ���ɫ�廯��������
���Կ���������ֱ����а�ɫ�������ɡ������������е���ɫ�������ɣ�
�ʴ�Ϊ����ɫ�������ɣ������������е���ɫ�������ɣ�
��2�������ӺͰ�ˮ��Ӧ������������������笠����ӣ����ӷ�Ӧ����ʽΪ��Ag++NH3?H2O�TAgOH��+NH4+��
���������Ͱ�ˮ��Ӧ����������Һ��ˮ�����ӷ�Ӧ����ʽΪ��AgOH+2NH3?H2O�TAg��NH3��2++OH?+2H2O��
���Կ����������ǣ������ɰ�ɫ�������ɫ��������ʧ��Һ����壬
�ʴ�Ϊ�������ɰ�ɫ�������ɫ��������ʧ��Һ����壻Ag++NH3?H2O�TAgOH��+NH4+��AgOH+2NH3?H2O�TAg��NH3��2++OH?+2H2O��
���Կ���������ֱ����а�ɫ�������ɡ������������е���ɫ�������ɣ�
�ʴ�Ϊ����ɫ�������ɣ������������е���ɫ�������ɣ�
��2�������ӺͰ�ˮ��Ӧ������������������笠����ӣ����ӷ�Ӧ����ʽΪ��Ag++NH3?H2O�TAgOH��+NH4+��
���������Ͱ�ˮ��Ӧ����������Һ��ˮ�����ӷ�Ӧ����ʽΪ��AgOH+2NH3?H2O�TAg��NH3��2++OH?+2H2O��
���Կ����������ǣ������ɰ�ɫ�������ɫ��������ʧ��Һ����壬
�ʴ�Ϊ�������ɰ�ɫ�������ɫ��������ʧ��Һ����壻Ag++NH3?H2O�TAgOH��+NH4+��AgOH+2NH3?H2O�TAg��NH3��2++OH?+2H2O��
���������⿼�������������ɼ����ʣ���ȷ����������ܲ��������ƶ������ӣ����Ƚ粻���������ƶ������ӣ�Ϊ�״��㣮

��ϰ��ϵ�д�
 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
�����Ŀ
 ���и�ѡ���мס��ҵĹ�ϵ������ʾ��ͼ��ʾ���ǣ������� ���и�ѡ���мס��ҵĹ�ϵ������ʾ��ͼ��ʾ���ǣ�������
|
������һ����Ҫ�Ļ�����Ʒ����������Ρ����صȵ�ԭ�ϣ���ҵ�ϳɰ��ķ�Ӧ���£�
N2��g��+3H2��g��?2NH3��g����H=-92.4kJ?mol-1��
��1��ʵ�����г������Ʊ������Ļ�ѧ����ʽΪ ��
��2����֪H2��g����ȼ����Ϊ285.8kJ?mol-1��д��NH3��g���ڴ�����ȼ���������������ʵ��Ȼ�ѧ����ʽ ��
��3��25��ʱ����a mol ��NH4��2SO4����ˮ�������Һ�еμ�V Lϡ��ˮ����Һ�����ԣ���μӰ�ˮ�Ĺ�����ˮ�ĵ���ƽ�⽫ ��������������������ƶ������μ�ϡ��ˮ�����ʵ���Ũ��Ϊ mol?L-1����25��ʱ��NH3?H2O�ĵ���ƽ�ⳣ��Kb��2��10-5����
��4����ҵ�ϳ���CO2��NH3ͨ�����·�Ӧ�ϳ�����[CO��NH2��2]��
CO2��g��+2NH3��g��
CO��NH2��2��l��+H2O��g����H��0��t��ʱ�����ݻ��㶨Ϊ2L���ܱ������м���0.10mol CO2��0.40mol NH3��70min��ʼ�ﵽƽ�⣮��Ӧ��CO2��g�������ʵ�����ʱ��仯���±���ʾ��
��20minʱv����CO2�� 80minʱv����H2O�������������=����������
����100minʱ�����������������䣬���������г���0.050mol CO2��0.20mol NH3�����½���ƽ���CO2��ת������ԭƽ����Ƚ� ������������䡱��С������
�۸��ݱ���������ͼ���л��Ƴ���t����NH3��ת������ʱ��仯��ͼ���������������䣬��t+10��������ȷ��ͼ������� ����ͼ���еġ�A����B������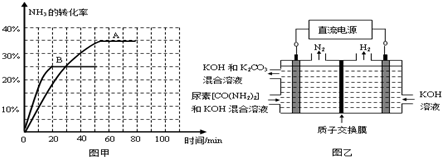
��ͼ����ʾװ�ã�����������Ϊ���Ե缫�������ڵ������[CO��NH2��2]�ļ�����Һ��ȡ��������װ���������ĵ缫��ӦʽΪ �����������ռ�������22.4L��������������ĵ�����Ϊ g������������ܽ⣩��
N2��g��+3H2��g��?2NH3��g����H=-92.4kJ?mol-1��
��1��ʵ�����г������Ʊ������Ļ�ѧ����ʽΪ
��2����֪H2��g����ȼ����Ϊ285.8kJ?mol-1��д��NH3��g���ڴ�����ȼ���������������ʵ��Ȼ�ѧ����ʽ
��3��25��ʱ����a mol ��NH4��2SO4����ˮ�������Һ�еμ�V Lϡ��ˮ����Һ�����ԣ���μӰ�ˮ�Ĺ�����ˮ�ĵ���ƽ�⽫
��4����ҵ�ϳ���CO2��NH3ͨ�����·�Ӧ�ϳ�����[CO��NH2��2]��
CO2��g��+2NH3��g��
| һ������ |
| ʱ��/min | 0 | 30 | 70 | 80 | 100 |
| n��CO2��/mol | 0.10 | 0.060 | 0.040 | 0.040 | 0.040 |
����100minʱ�����������������䣬���������г���0.050mol CO2��0.20mol NH3�����½���ƽ���CO2��ת������ԭƽ����Ƚ�
�۸��ݱ���������ͼ���л��Ƴ���t����NH3��ת������ʱ��仯��ͼ���������������䣬��t+10��������ȷ��ͼ�������

��ͼ����ʾװ�ã�����������Ϊ���Ե缫�������ڵ������[CO��NH2��2]�ļ�����Һ��ȡ��������װ���������ĵ缫��ӦʽΪ
