��Ŀ����
��һ������Ͼ��ȵ�����������ڸ��������������¹��ȣ���ַ�Ӧ����ȴ�����£��õ�����A��������Ϊm�Ĺ���A���뵽300 mL 2 mol��L-1������ʹ֮��ȫ�ܽ⣬��������¼������A���������ռ���������������ѻ���ɱ�״�����Ĺ�ϵ����ͼ��ʾ������������������Һ����ǰ�����������ݳ�����
��֪�������A������m��3.2 gʱ���ռ���������Ϊ��������m��3.2 gʱ���ռ���������ΪH2��H2S�Ļ�����塣
�Է������㣺
��1��3.2 g����A��������������_____________��
��2��3.2 g����A�и����ʵ����ʵ����ֱ�Ϊ_______________���������������ַ�Ӧ��������Һ������������ʵ���Ũ��Ϊ��������Һ����ı仯��______________��
��3��������Aȫ�������������ᣬ��A������m��3.2 gʱ���ռ�������������������V=____________mL(�ú�m�Ĵ���ʽ��ʾ����
��1��FeS��Fe
(2)FeΪ0.56 g,FeSΪ2.64 g 0.1 mol��L-1
(3)280m-672
��������1������������ڸ������������¹��Ⱥ������ɵIJ���������������������һ��FeS��S������FeS��Fe������FeS�������⣬������뵽300 mL 2 mol��L-1�������ܹ���ȫ�ܽ⣬�ɵù���A�в����ڵ������ų��˵�һ��������������⣬��m��3.2 gʱ���ռ�������ΪH2��H2S�Ļ�������ɵó�����A��FeS��Fe�Ļ�����2��������ͼ��֪�ڹ���A������m��3.2 gʱ���ռ���������ֻ��H2����ǰ�����ɵ�H2Sȫ������ˮ�У�Ҳ����m=3.2 gʱ��H2S��ˮ�е��ܽ�ﵽ����״̬������Fe��H2�ɼ������������Ϊ0.56 g,��ôFeS������Ϊ2.64 g��������H2S�����ʵ���Ϊn(H2S)=n(FeS)=![]() =0.03 mol������������ʵ���Ũ��Ϊ��c(H2S)=
=0.03 mol������������ʵ���Ũ��Ϊ��c(H2S)=![]() =0.1 mol��L-1����3���������⣬�ų�����Ķ�������Ԫ�ص����ʵ����������ɣ�2����֪A��Fe����������Ϊ
=0.1 mol��L-1����3���������⣬�ų�����Ķ�������Ԫ�ص����ʵ����������ɣ�2����֪A��Fe����������Ϊ![]() ��100%=70%��m g A�к��������ʵ���Ϊ
��100%=70%��m g A�к��������ʵ���Ϊ![]() =0.0125m mol,�ų�����280m mL��������0.03 mol H2S����HCl�У���ų�����Ϊ(280m-672)mL��
=0.0125m mol,�ų�����280m mL��������0.03 mol H2S����HCl�У���ų�����Ϊ(280m-672)mL��

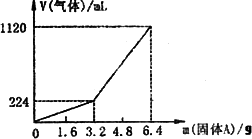
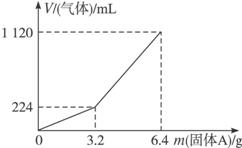
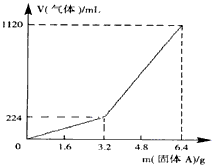
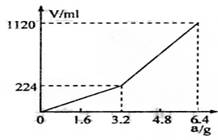 300mL2mol?L��1������ʹ֮��ȫ�ܽ⡣�ռ�����������V����״���²ⶨ����������A������a֮��Ĺ�ϵ��ͼ��ʾ��������������������Һ����ǰ�����������ݳ�������֪�������A������a��3.2gʱ���ռ���������Ϊ��������a>3.2g���ռ���������ΪH2��H2S�Ļ�������Է�������㣺
300mL2mol?L��1������ʹ֮��ȫ�ܽ⡣�ռ�����������V����״���²ⶨ����������A������a֮��Ĺ�ϵ��ͼ��ʾ��������������������Һ����ǰ�����������ݳ�������֪�������A������a��3.2gʱ���ռ���������Ϊ��������a>3.2g���ռ���������ΪH2��H2S�Ļ�������Է�������㣺