��Ŀ����
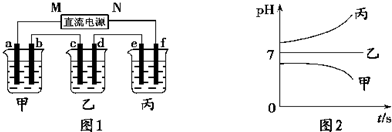
��A��B��C���ֵ������Һ�ֱ�װ�������ձ��У�����ʯī�缫������ͼ3-4��ʾ��ʽ�ڵ�·�����ӡ��պϿ���K��ø�֧·����ǿ�Ȣ����֢���(���Т�����С)�� 
ͼ3-4
����ȥB����֪����ǿ�Ȣ�A������C������ȥC������A��B����Һ���Ⱥ����Ϊ���ȷݣ��������ڵ�·����֪ͨ��A��B�����Һ�ĵ���ǿ������ǰͨ��A��Һ�ĵ���ǿ�ȵ���Դ�С��ϵΪ����sAB������A
��֪A��B��C�ֱ�ѡ��������Һ��
��0.1 mol��L-1���� ��0.1 mol��L-1���� ��0.1 mol��L-1NaCl��Һ ��0.1 mol��L-1���� ��0.1 mol��L-1 NaOH��Һ ��0.1 mol��L-1��ˮ����25 ��ʱ��A��ҺpH��7
�ش��������⣺
(1)ָ��A��B��C��(�������)ʲô��Һ?
A____________��B____________��C____________��
(2)����C��Һ�е����̪�Լ��ʺ�ɫ����C��____________����A��B��C�ֱ��Ե��������������ϣ������������ϵĻ��Һ�У�ˮ�ĵ���̶����? ____________��(ѡ�A����B����C��)
˼·���������⿼��ǿ��������뵼���ԵĹ�ϵ�����ȸ�����·���ӷ�ʽ��B��C������A��B��C�������ٸ���ʵ����������֪A��B��һ����������ʶ�C��Ϊǿ����ʡ�����ΪA��ҺpH��7�ɵô𰸡�
�𰸣�(1)0.1 mol��L-1���� 0.1 mol��L-1��ˮ �������־��� (2)NaOH A��B���Һ