��Ŀ����
��ҵ�Ͽ���ʳ�κ�ʯ��ʯΪ��Ҫԭ�ϣ�����ͬ�ķ������������ش��������⣺
��1��¬����������ʳ�Ρ�ʯ��ʯ��Ũ���ᡢ��̿Ϊԭ�ϣ��ڸ����½������գ��ٽ�ȡ���ᾧ���Ƶô��
��ʳ�κ�Ũ���ᷴӦ�Ļ�ѧ����ʽΪ___________��
�������ƺͽ�̿��ʯ��ʯ��Ӧ�Ļ�ѧ����ʽΪ_________����֪����֮һΪCaS����
��2������Ĺ�������ͼ��ʾ���õ���̼�����ƾ��������ɴ��
| | |
| |  |
��װ�����з�����Ӧ�Ļ�ѧ����ʽΪ_______��

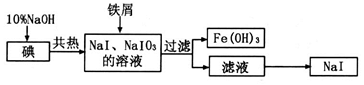
��3�������Ƽ����ĸĽ������ŵ���______________��
��4��������Ϊ̼�������̼�����ƵĻ�ѧ�������ƣ���Ҳ���ð�����Ȼ��غ�ʯ��ʯΪԭ����̼��ء�������ͼ���ܽ�ȣ�S�����¶ȱ仯���ߣ�����˵���Ƿ���У�__________��
��1����2NaCl+H2SO4(Ũ)  Na2SO4+2HCl��
Na2SO4+2HCl��
��Na2SO4+4C+CaCO3 CaS+Na2CO3+4CO��
CaS+Na2CO3+4CO��
Na2SO4+2C+CaCO3 CaS+Na2CO3+2CO2
CaS+Na2CO3+2CO2
��2����Ca��OH��2 NH3
��NH3+CO2+NaCl+H2O=NaHCO3��+NH4Cl
��3�������ԭ�ϵ������ʣ����ٷ�����CaCl2�����ŷţ������˰�����ŵ㣬����������ȱ�㣬ʹʳ�ε���������ߣ�NH4Cl �������ʣ�����ϳɰ������ϣ�ʹ�ϳɰ���ԭ���� CO ת���� CO2������ CaCO3�� CO2��һ����
��4�������У���ΪKHCO3��NH4Cl���ܽ�������¶ȸ���40��ʱ��KHCO3���ܽ�ȴ���NH4Cl�����½ᾧʱ�������϶��KCl��
 Na2SO4+2HCl��
Na2SO4+2HCl����Na2SO4+4C+CaCO3
 CaS+Na2CO3+4CO��
CaS+Na2CO3+4CO��Na2SO4+2C+CaCO3
 CaS+Na2CO3+2CO2
CaS+Na2CO3+2CO2��2����Ca��OH��2 NH3
��NH3+CO2+NaCl+H2O=NaHCO3��+NH4Cl
��3�������ԭ�ϵ������ʣ����ٷ�����CaCl2�����ŷţ������˰�����ŵ㣬����������ȱ�㣬ʹʳ�ε���������ߣ�NH4Cl �������ʣ�����ϳɰ������ϣ�ʹ�ϳɰ���ԭ���� CO ת���� CO2������ CaCO3�� CO2��һ����
��4�������У���ΪKHCO3��NH4Cl���ܽ�������¶ȸ���40��ʱ��KHCO3���ܽ�ȴ���NH4Cl�����½ᾧʱ�������϶��KCl��
��1������Ŀ��Ϣ��֪���� ʳ����Ũ����������������ƺ�HCl�������������������ƺ�HCl��2NaCl+H2SO4(Ũ)  Na2SO4+2HCl������������ʯ��ʯ����̿��Ӧ����CaS��Na2CO3������Ԫ���غ��֪����ԭC��������CO��CO2���ɣ�
Na2SO4+2HCl������������ʯ��ʯ����̿��Ӧ����CaS��Na2CO3������Ԫ���غ��֪����ԭC��������CO��CO2���ɣ�
�ʷ�Ӧ����ʽΪ��Na2SO4+4C+CaCO3 CaS+Na2CO3+4CO
CaS+Na2CO3+4CO
��Na2SO4+2C+CaCO3 CaS+Na2CO3+2CO2
CaS+Na2CO3+2CO2
��2����̼��Ƽ��ȷֽ�����CaO��CO2����AΪCaO��BΪCO2��CaO���컯Ͱ����ˮ��Ӧ�����������ƣ���CΪCa��OH��2�������������Ȼ���ڻ�ϳ��ڷ�Ӧ���ɰ������Ȼ��ƣ���DΪNH3��
�ڡ��������Ȼ�����Һ��Ϻ��������������̼������Ӧ������NaHCO3��NH4Cl����Ӧ����ʽΪNH3+CO2+NaCl+H2O=NaHCO3+NH4Cl��
��3�������ԭ�ϵ������ʣ����ٷ�����CaCl2�����ŷţ������˰�����ŵ㣬����������ȱ�㣬ʹʳ�ε���������ߣ�NH4Cl �������ʣ�����ϳɰ������ϣ�ʹ�ϳɰ���ԭ���� CO ת���� CO2������� CaCO3�� CO2��һ����
��4�������У���ΪKHCO3��NH4Cl���ܽ�������¶ȸ���40��ʱ��KHCO3���ܽ�ȴ���NH4Cl�����½ᾧʱ�������϶��KCl��
�����У���ΪKHCO3��NH4Cl���ܽ�������¶ȸ���40��ʱ��KHCO3���ܽ�ȴ���NH4Cl�����½ᾧʱ�������϶��KCl
 Na2SO4+2HCl������������ʯ��ʯ����̿��Ӧ����CaS��Na2CO3������Ԫ���غ��֪����ԭC��������CO��CO2���ɣ�
Na2SO4+2HCl������������ʯ��ʯ����̿��Ӧ����CaS��Na2CO3������Ԫ���غ��֪����ԭC��������CO��CO2���ɣ��ʷ�Ӧ����ʽΪ��Na2SO4+4C+CaCO3
 CaS+Na2CO3+4CO
CaS+Na2CO3+4CO��Na2SO4+2C+CaCO3
 CaS+Na2CO3+2CO2
CaS+Na2CO3+2CO2��2����̼��Ƽ��ȷֽ�����CaO��CO2����AΪCaO��BΪCO2��CaO���컯Ͱ����ˮ��Ӧ�����������ƣ���CΪCa��OH��2�������������Ȼ���ڻ�ϳ��ڷ�Ӧ���ɰ������Ȼ��ƣ���DΪNH3��
�ڡ��������Ȼ�����Һ��Ϻ��������������̼������Ӧ������NaHCO3��NH4Cl����Ӧ����ʽΪNH3+CO2+NaCl+H2O=NaHCO3+NH4Cl��
��3�������ԭ�ϵ������ʣ����ٷ�����CaCl2�����ŷţ������˰�����ŵ㣬����������ȱ�㣬ʹʳ�ε���������ߣ�NH4Cl �������ʣ�����ϳɰ������ϣ�ʹ�ϳɰ���ԭ���� CO ת���� CO2������� CaCO3�� CO2��һ����
��4�������У���ΪKHCO3��NH4Cl���ܽ�������¶ȸ���40��ʱ��KHCO3���ܽ�ȴ���NH4Cl�����½ᾧʱ�������϶��KCl��
�����У���ΪKHCO3��NH4Cl���ܽ�������¶ȸ���40��ʱ��KHCO3���ܽ�ȴ���NH4Cl�����½ᾧʱ�������϶��KCl

��ϰ��ϵ�д�
�����Ŀ



 �ȸ���Ӧ��
�ȸ���Ӧ��



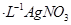 ��Һ�ζ����յ㣬����
��Һ�ζ����յ㣬����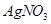 ��Һ�����ƽ��ֵΪ19��00mL��
��Һ�����ƽ��ֵΪ19��00mL��