��Ŀ����
��֪25��ʱ��
��HF(aq)��OH��(aq)=F��(aq)��H2O(l)����H1����67.7 kJ��mol��1
��H��(aq)��OH��(aq)=H2O(l)����H2����57.3 kJ��mol��1
��Ba2��(aq)��SO42��(aq)=BaSO4(s)����H3��0
����˵����ȷ����(����)
| A��HF�ĵ��뷽��ʽ����ЧӦ��HF(aq)=H��(aq)��F��(aq)����H��0 |
| B��������������Һ������ķ�Ӧ�У�������һ��������������Һ��Խ�࣬�к���Խ�� |
| C����H2����57.3 kJ��mol��1��ǿ���ǿ����ϡ��Һ�з�Ӧ���ɿ����ε��к��� |
| D��ϡ������ϡ����������Һ��Ӧ���Ȼ�ѧ����ʽΪH2SO4(aq)��Ba(OH)2(aq)=BaSO4(s)��2H2O(l)��H����114.6 kJ��mol��1 |
C
����

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д����й����������ų�����
| A���Ͽ������е�H��H�� |
| B��C��H2O(g)��Ӧ |
| C��Ba(OH)2��8H2O������NH4Cl���巴Ӧ |
| D��Na��H2O��Ӧ |
����ͼʾ���Ӧ�������������
| A��ͼ1��ʾ��ij����������������Һ�м���NaOH��Һ���������������NaOH��Һ����Ĺ�ϵ |
| B��ͼ2��ʾ25��ʱ����0.1mol?L-1����ζ�20mL0.1mol?L-1NaOH��Һ��pH�������������ı仯 |
| C��ͼ3��ʾt��ʱϡ�ͱ������������Һ�����Եı仯 |
| D������ͼ4���ж�ij���淴Ӧ������Ӧ�����ȷ�Ӧ |
��˹������Ϊ�����ܻ�ѧ������һ�����Ϊ������ɣ�������̵���ЧӦ����ͬ�ġ�
��֪��H2O��g���� H2O��l�� ��H1 ����Q1 kJ��mol��1��Q1��0��
C2H5OH��g���� C2H5OH��l�� ��H2 ����Q2 kJ��mol��1��Q2��0��
C2H5OH��g����3O2��g����2CO2��g����3H2O��g�� ��H3 �� ��Q3 kJ��mol��1��Q3��0����ʹ23gҺ̬�Ҵ���ȫȼ�գ����ָ������£���ų�������Ϊ��kJ��
| A��Q1�� Q2��Q3 | B��0.5��Q1��Q2��Q3 �� |
| C��0.5 Q1��1.5 Q2��0.5Q3 | D��1.5 Q1��0.5 Q2��0.5Q3 |
��֪�Ͽ����γ�1 mol��ѧ�����ջ�ų���������Ϊ��ѧ���ļ��ܣ���H��H���ļ���Ϊ436 kJ��mol��1��N��N���ļ���Ϊ945 kJ��mol��1��N��H���ļ���Ϊ391 kJ��mol��1���������йع�ҵ�ϳɰ���Ӧ���Ȼ�ѧ����ʽ��ȷ���� (����)
A��N2(g)��3H2(g)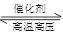 2NH3(g)����H����93 kJ��mol��1 2NH3(g)����H����93 kJ��mol��1 |
B��N2(g)��3H2(g)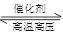 2NH3(g)����H����1471 kJ��mol��1 2NH3(g)����H����1471 kJ��mol��1 |
C��N2(g)��3H2(g)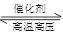 2NH3(g)����H����93 kJ��mol��1 2NH3(g)����H����93 kJ��mol��1 |
D��N2(g)��3H2(g)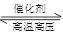 2NH3(g)����H����1471 kJ��mol��1 2NH3(g)����H����1471 kJ��mol��1 |
��ѧ��ӦA2��B2=2AB�������仯��ͼ��ʾ��������˵����ȷ���ǣ� ��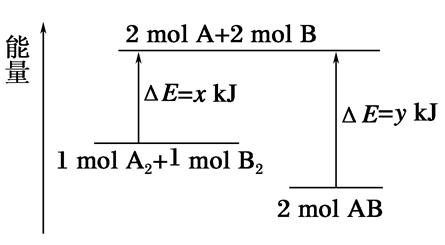
| A���÷�Ӧ�����ȷ�Ӧ |
| B������1mol A��A����1mol B��B���ܷų�xkJ������ |
| C������2mol A��B����Ҫ����ykJ������ |
| D��2mol AB������������1 mol A2��1mol B2�������� |
���ݵ���������Ӧ���Ȼ�ѧ����ʽ
(��)I2(g)��H2(g)  2HI(g)����H����9��48 kJ��mol��1
2HI(g)����H����9��48 kJ��mol��1
(��)I2(s)��H2(g)  2HI(g)����H����26��48 kJ��mol��1
2HI(g)����H����26��48 kJ��mol��1
�����ж���ȷ���ǣ� ��
| A��1 mol I2(s)��ͨ��2 g H2(g)����Ӧ����26��48 kJ |
| B��1 mol��̬����1 mol��̬���������������17��00 kJ |
| C����Ӧ(��)�ķ�Ӧ���������ȷ�Ӧ(��)�ķ�Ӧ���������� |
| D����Ӧ(��)�ų��������࣬���Բ���������ͣ��ȷ�Ӧ(��)�IJ�����ȶ� |
��֪����ȼ�����ɶ�����̼��Һ̬ˮ�ų�������Ϊ55.625 kJ��g-1�������Ȼ�ѧ����ʽ�в���ȷ����(����)
A��CH4(g)+2O2(g) CO2(g)+2H2O(l) ��H ="-890" kJ/mol CO2(g)+2H2O(l) ��H ="-890" kJ/mol |
B��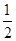 CH4(g)+O2(g) CH4(g)+O2(g) 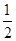 CO2(g)+H2O(l) ��H="-445" kJ/mol CO2(g)+H2O(l) ��H="-445" kJ/mol |
C��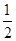 CO2(g)+H2O(l) CO2(g)+H2O(l) 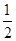 CH4(g)+O2(g) ��H="+445" kJ/mol CH4(g)+O2(g) ��H="+445" kJ/mol |
D��CH4(g)+2O2(g) CO2(g)+2H2O(l) ��H="-55.625" kJ/mol CO2(g)+2H2O(l) ��H="-55.625" kJ/mol |