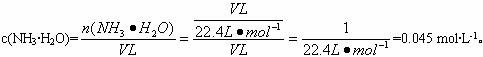��Ŀ����
 ʵ������ȡ�����м��ַ�����
ʵ������ȡ�����м��ַ�������1������NH4Cl��Ca��OH��2����ȡ����ʱӦѡ��װ����
A
A
���䷴Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O
| ||
2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O
��
| ||
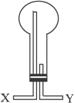
��2���ռ�����ʱӦѡ��
��������
��������
������3��д�����鰱���Ƿ��Ѿ��ռ����ķ�����
��һ��ʪ��ĺ�ɫʯ����ֽ�ӽ��Թܿڣ���ֽ����˵�����ռ���
��һ��ʪ��ĺ�ɫʯ����ֽ�ӽ��Թܿڣ���ֽ����˵�����ռ���
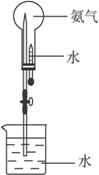
��4��Ϊ�˵õ������NH3����
A
A
�������A����ʯ�� B��ŨH2SO4 C����ˮCaCl2 D��P2O5��
��������1�����ݷ�Ӧ��״̬�ͷ�Ӧ���������ж��Ʊ������װ�ã���Ӧ�ǹ����Ȼ�狀��������Ƽ��ȷ�Ӧ���ɰ�����
��2���������ܶȱȿ���С��
��3�����ݰ�����ʪ��ĺ�ɫʯ����ֽ����֤����
��4�������Ǽ��������ü��Ը�������
��2���������ܶȱȿ���С��
��3�����ݰ�����ʪ��ĺ�ɫʯ����ֽ����֤����
��4�������Ǽ��������ü��Ը�������
����⣺��1����Ӧ���ǹ����������Ʊ�����ķ�Ӧ��ѡ�ô��Թܼ����Ʊ�������ѡ��װ��A���ù����Ȼ�狀��������Ƽ��ȷ�Ӧ���ɰ�������Ӧ�Ļ�ѧ����ʽ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��A��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
��2�������ܶȱȿ����ᣬ������ˮ���������ſ������ռ���
�ʴ𰸣���������������
��3�����鰱���Ƿ��Ѿ��ռ����ķ�������һ��ʪ��ĺ�ɫʯ����ֽ�ӽ��Թܿڣ���ֽ����˵�����ռ�����
�ʴ�Ϊ����һ��ʪ��ĺ�ɫʯ����ֽ�ӽ��Թܿڣ���ֽ����˵�����ռ�����
��4�����ﰱ����Ҫѡ����Ը������
A����ʯ�� �Ǽ��Ը�������Ը��ﰱ������A���ϣ�
B��ŨH2SO4 �Ͱ�����Ӧ�������������ﰱ������B�����ϣ�
C����ˮCaCl2�Ͱ�����Ӧ�����������ܸ��ﰱ������C�����ϣ�
D��P2O5��ˮ��Ӧ��������������հ��������ܸ��ﰱ������D�����ϣ�
��ѡ��A��
| ||
�ʴ�Ϊ��A��2NH4Cl+Ca��OH��2
| ||
��2�������ܶȱȿ����ᣬ������ˮ���������ſ������ռ���
�ʴ𰸣���������������
��3�����鰱���Ƿ��Ѿ��ռ����ķ�������һ��ʪ��ĺ�ɫʯ����ֽ�ӽ��Թܿڣ���ֽ����˵�����ռ�����
�ʴ�Ϊ����һ��ʪ��ĺ�ɫʯ����ֽ�ӽ��Թܿڣ���ֽ����˵�����ռ�����
��4�����ﰱ����Ҫѡ����Ը������
A����ʯ�� �Ǽ��Ը�������Ը��ﰱ������A���ϣ�
B��ŨH2SO4 �Ͱ�����Ӧ�������������ﰱ������B�����ϣ�
C����ˮCaCl2�Ͱ�����Ӧ�����������ܸ��ﰱ������C�����ϣ�
D��P2O5��ˮ��Ӧ��������������հ��������ܸ��ﰱ������D�����ϣ�
��ѡ��A��
���������⿼����ʵ���Ұ������Ʊ�װ�ú��Լ�ѡ��Ӧԭ���ķ����жϣ����ջ����ǽ���ؼ�����Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ
��ij��ѧ��ȤС���ͬѧ�����Ƶ���ˮ��������ʵ�飺������ˮ�е���Na2CO3��Һ��������ˮ�е���AgNO3��Һ��������ˮ�е�����ɫʯ����Һ�����۲쵽��ʵ���������£��뽫��Ӧ��ʵ����������±��ո��У�
��С���ͬѧΪ̽����������ȡ�������ʣ�����������ʵ�飮
��1����ʵ�������������з����Ʊ����������в��������ǣ�����ţ� ��
A������Ũ��ˮ
B������ʯ�Һ��Ȼ�淋Ļ�������
C����Ũ��ˮ�ε���ʯ����
D�������Ȼ�粒���
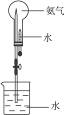
��2������������ȷ�ķ�����ѡ��ͼ��ʾ��װ����ȡ������д���Թ�����������Ӧ�Ļ�ѧ����ʽ ��һ��ʱ���һ��պ��Ũ����IJ������ӽ����ܿڣ��۲쵽�������� ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
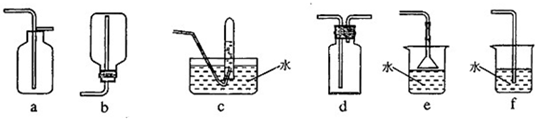
��3��ͬѧ����Ƶ��ռ������հ����ļ���װ������ͼ��ʾ�������������ռ��������ǣ�����ţ� �����������հ���β�����ǣ�����ţ� ��
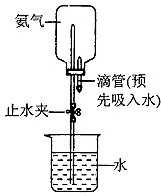
��4�������ռ��������ļ���ƿ����ͼ��ʾ���Ӻ�װ�ã��г�װ�ü���ȥ��������Ȫʵ��ʱ����д������ˮ����IJ����� ��
| ʵ����� | ʵ������ |
| ��Һ�ȱ�����ɫ | |
| �а�ɫ�������� | |
| �����ݲ�������ˮ��ɫ��dz |
��1����ʵ�������������з����Ʊ����������в��������ǣ�����ţ�
A������Ũ��ˮ
B������ʯ�Һ��Ȼ�淋Ļ�������
C����Ũ��ˮ�ε���ʯ����
D�������Ȼ�粒���
��2������������ȷ�ķ�����ѡ��ͼ��ʾ��װ����ȡ������д���Թ�����������Ӧ�Ļ�ѧ����ʽ

��3��ͬѧ����Ƶ��ռ������հ����ļ���װ������ͼ��ʾ�������������ռ��������ǣ�����ţ�

��4�������ռ��������ļ���ƿ����ͼ��ʾ���Ӻ�װ�ã��г�װ�ü���ȥ��������Ȫʵ��ʱ����д������ˮ����IJ�����


 �ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��
�ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��