��Ŀ����
13��ij����С���ú�����þ��������ȡ��������������������ͼ��
��1��д������������������ռ���Һ������Ӧ�����ӷ���ʽ2Al+2OH-+2H2O�T2AlO2-+3H2����
��2���ڢٲ���ʵ���������Ϊ���ˣ�
��3���ڢڲ������õ���ҺA�м����ͨ�����й�����D���Ի�Al��OH��3������
A��ϡ���� B���ռ���Һ C����ˮ D��CO2
��4���ڵڢ۲�ʵ���������Ϊϴ�ӳ�����
��5���ڢܲ�ʵ���������Ϊ���ȣ�������Ӧ�Ļ�ѧ����ʽΪ2Al��OH��3�TAl2O3+3H2O��
���� ����þ������ѧ���ʵIJ��죺��������Ӧ����þ��������ķ�����ʹ����������������Һ������Bþ���ܽ⣬���˷��룬�ٽ���ҺAƫ�����������ᷴӦ��Ϊ����C���������������������ȷֽ�õ�������������
��� �⣺����þ������ѧ���ʵIJ��죺��������Ӧ����þ��������ķ�����ʹ����������������Һ������Bþ���ܽ⣬���˷��룬�ٽ���ҺAƫ�����������ᷴӦ��Ϊ����C���������������������ȷֽ�õ�������������
��1�������������Ʒ�Ӧ����ƫ�����ƺ����������ӷ���ʽ2Al+2OH-+2H2O�T2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
��2�������Һ������ù��ˣ��ʴ�Ϊ�����ˣ�
��3��ƫ���������ᷴӦ�ɵõ����������������������������ᣬ��ֻ���ö�����̼���ʴ�Ϊ��D��
��4��ϴ�ӳ����������������ʣ��ʴ�Ϊ��ϴ�ӳ�����
��5���������������ɵõ���������������ѧ����ʽΪ2Al��OH��3�TAl2O3+3H2O���ʴ�Ϊ�����ȣ�2Al��OH��3�TAl2O3+3H2O��
���� ���⿼�����ʵķ��롢�ᴿ��������ѧ���ķ���������ʵ�����������������Ŀ��飬�ѶȲ���ע��������ʵ����ʵ���ͬ��ע�����ʱ���������µ����ʣ�

 �Ķ��쳵ϵ�д�
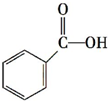
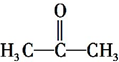
�Ķ��쳵ϵ�д�| A�� | CH3CH=C��CH3�� CH3 3-��-2-��ϩ | |
| B�� | CH3CH��CH3��CH��Cl��CH3 3-��-2-�ȶ��� | |
| C�� | CH3CH��OH��CH2CH3 2-�ǻ����� | |
| D�� | CH3CH��C2H5��CH2CH2CH3 2-�һ����� |
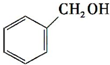
| A�� | CH2=CH2+H-OH$\frac{\underline{����}}{��}$CH3-CH2-OH | |
| B�� | CH2=CH-CH=CH2+2H2$\frac{\underline{\;����\;}}{\;}$CH3-CH2-CH2-CH3 | |
| C�� |  +H2$\stackrel{����}{��}$CH3-CH2-OH +H2$\stackrel{����}{��}$CH3-CH2-OH | |
| D�� | CH3-CH3+2Cl2$\stackrel{����}{��}$CH2Cl-CH2Cl+2HCl |
| A�� | 2g H2���е���ԭ����ĿΪNA | |
| B�� | ���³�ѹ�£�22.4 LO2���еķ�����ĿΪNA | |
| C�� | 1 molZnת��ΪZn2+ʧȥ�ĵ�����ĿΪNA | |
| D�� | 1 L 1mol•L-1KOH��Һ�к��еļ�������ĿΪNA |
 ijͬѧ�Ը�����Ϊԭ����ˮ��һ����һˮ��ѭ��������ȡ���Ტ̽���ⶨ���ᾧ�壨H2C2O4•xH2O����ijЩ���ʣ�ͨ���������Ͽ�֪������������ˮ����һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᣮ157��ʱ��������������ʼ�ֽ⣻���������ڵ����¿�����Ϊ���壻����Ʋ�����ˮ������������ʹ����ʯ��ˮ����ǣ�
ijͬѧ�Ը�����Ϊԭ����ˮ��һ����һˮ��ѭ��������ȡ���Ტ̽���ⶨ���ᾧ�壨H2C2O4•xH2O����ijЩ���ʣ�ͨ���������Ͽ�֪������������ˮ����һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᣮ157��ʱ��������������ʼ�ֽ⣻���������ڵ����¿�����Ϊ���壻����Ʋ�����ˮ������������ʹ����ʯ��ˮ����ǣ�

 Cl2��H2O2�Ǹ��н����������������������������Cl2��������ǿ��H2O2���ܽ�H2O2������Ϊ����֤�ý��ۣ�ѧ���������ͼ��ʾ��ʵ��װ�ý���ʵ�飨�г�װ����ȥ������ش��������⣺
Cl2��H2O2�Ǹ��н����������������������������Cl2��������ǿ��H2O2���ܽ�H2O2������Ϊ����֤�ý��ۣ�ѧ���������ͼ��ʾ��ʵ��װ�ý���ʵ�飨�г�װ����ȥ������ش��������⣺ HCl+HClO��2HClO$\frac{\underline{\;����\;}}{\;}$2HCl+O2�������÷���ʽ�ش𣩣��������ɿ��Բ��öԱ�ʵ������
HCl+HClO��2HClO$\frac{\underline{\;����\;}}{\;}$2HCl+O2�������÷���ʽ�ش𣩣��������ɿ��Բ��öԱ�ʵ������ ��Ԥ��÷������ܣ���ܡ����ܡ����������෴Ӧ��
��Ԥ��÷������ܣ���ܡ����ܡ����������෴Ӧ��
 ��c��
��c��