��Ŀ����
13�� ��������AIN����һ�����͵������ϣ��㷺Ӧ���ڼ��ɵ�·��������������Ʒ����������̿�����������ʣ���ѧ�о���ѧϰС��Ϊ�ⶨ�ò�Ʒ�е�����������������ȡһ�����ĵ�������Ʒ����������ʵ�飮��֪��
��������AIN����һ�����͵������ϣ��㷺Ӧ���ڼ��ɵ�·��������������Ʒ����������̿�����������ʣ���ѧ�о���ѧϰС��Ϊ�ⶨ�ò�Ʒ�е�����������������ȡһ�����ĵ�������Ʒ����������ʵ�飮��֪��AlN+NaOH+H2O=NaAlO2+NH3����
ͬѧ����Ƶ�ʵ��װ����ͼ��
��1�����ƿ��ˮ�Ϸ����Լ�X��ѡ��d��������ĸ����
a���ƾ� b���� c�����Ȼ�̼ d��ֲ����
��2�����ƿ�е�Һ��δװ�����Ϸ����пռ䣩��ʵ���õ�NH3�������c ������ĸ����
a��ƫ�� b��ƫС c������Ӱ��
��3��������A��������ƽ���Һ©���Ϸ�����ƿ��ѹǿ��ʹҺ�������¡�ʹ���µ�Һ�������Ӱ��ⶨ��NH3�������
��4��ʵ����ȡ�õ�������Ʒm g��ʵ���ð������Ϊn L��������ɱ�״̬������õ�������Ʒ�е���������Ϊ$\frac{41n}{22.4m}$��
���� ��Ϊ���������������Ʒ�Ӧ�����ɰ���������������ƿ�����װ���ܱգ����ƿ��ѹǿ��������ô�ͻ���ˮͨ�����ƿ�ij��ܽ�����Ͳ�У����ݵ�������֪�����뵽���ƿ��ˮ������͵������ɵİ����������
��1��ˮ�Ϸ���Һ��������ˮ�������ܶȱ�ˮС��
��2��������ƿ�а���������������Ž���Ͳ��ˮ�������
��3��������A��ͨ��Һ©���Ϸ�����ƿʹ����ѹǿ��ͬ��
��4��ͨ����Ͳ���ų���ˮ������Ϳ���֪��������������������ݷ���ʽ�����������������
��� �⣺��1����Ϊ������������ˮ�����Թ��ƿ��ˮ����Ӧ��һ�����ʣ����������ˮ�������ƾ�������ˮ�����Ȼ�̼���²㣬Ӧѡ��ֲ���ͣ�
�ʴ�Ϊ��D��
��2�����ƿ�е���ѹ֮���Ի���������Ϊ�а������룬�������۹��ƿ���Ƿ��п���������ƿ�а���������������Ž���Ͳ��ˮ����������Բ���Ӱ�죬
�ʴ�Ϊ��C��
��3��������A��ͨ��Һ©���Ϸ�����ƿ����ʹ����ѹǿһ�£�����ƽ���Һ©���Ϸ�����ƿ��ѹǿ��ʹҺ�������£���������ϵ��ѹǿ�����ѹǿһ�£����Կ���ʹ���µ�Һ�������Ӱ��ⶨ��NH3�������
�ʴ�Ϊ��ƽ���Һ©���Ϸ�����ƿ��ѹǿ��ʹҺ�������£�ʹ���µ�Һ�������Ӱ��ⶨ��NH3�������
��4����μӷ�Ӧ��AlN����Ϊx
AlN+NaOH+H2O=NaAlO2+NH3��
41g 1mol
xg $\frac{nL}{22.4L/mol}$
��x=$\frac{41n}{22.4}$g��
����������$\frac{\frac{41n}{22.4}}{m}$=$\frac{41n}{22.4m}$��
�ʴ�Ϊ��$\frac{41n}{22.4m}$��
���� ���⿼�������ʺ����IJⶨ��ʵ����������ȣ������ڿ���ѧ����ʵ��̽�����������ݴ�����������Ŀ�Ѷ��еȣ�

| A�� | NH4+��Cl-��Na+��SO42- | B�� | Na+��Ba2+��OH-��SO42- | ||
| C�� | Ca2+��H+��S2-��ClO- | D�� | H+��Cl-��Fe2+��NO3- |
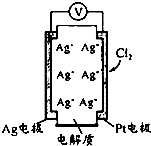 ������ͼ��ʾԭ��ؿɲ���������Cl2���������е������Ag+���������ƶ��Ĺ������ʣ����з�������ȷ���ǣ�������
������ͼ��ʾԭ��ؿɲ���������Cl2���������е������Ag+���������ƶ��Ĺ������ʣ����з�������ȷ���ǣ�������| A�� | ���Ӿ����·����Pt�缫 | |
| B�� | ������Ӧ��Cl2+2e-+2Ag+=2AgCl | |
| C�� | ��ع���ʱ���������Ag+��Ŀ���� | |
| D�� | ������c��Cl2��Խ��Ag����������Խ�� |
| A�� | KClO3�������� | |
| B�� | �������뱻��ԭ����Ԫ�ص�������Ϊ5��1 | |
| C�� | H2SO4�Ȳ����������ֲ��ǻ�ԭ�� | |
| D�� | 1 mol KClO3�μӷ�Ӧʱ��10 mol����ת�� |
| A�� | HX | B�� | H2X | C�� | XH4 | D�� | XH3 |
C2H5Br��CH2=CH2��C1H2C-CH2Cl��CH2=CHCl��

| A�� | ��ȥ��Ӧ | B�� | ȡ����Ӧ | C�� | �ӳɷ�Ӧ | D�� | �Ӿ۷�Ӧ |
| A�� | c��K+��+c��H+��=c��HC2O4-��+c��OH-��+c��C2O42-�� | B�� | c��HC2O4-��+c��C2O42-��=0.1mol•L-1 | ||
| C�� | c��C2O42-����c��H2C2O4�� | D�� | c��K+��=c��H2C2O4��+c��HC2O4-��+c��C2O42-�� |
 ������ģ�ҵ�ϸ�����������������ͭ����Ʒ����Ҫ�ɷ�ΪCu2S��Cu2O�Ļ���ұ��ͭ��ʵ��װ��ͼ��
������ģ�ҵ�ϸ�����������������ͭ����Ʒ����Ҫ�ɷ�ΪCu2S��Cu2O�Ļ���ұ��ͭ��ʵ��װ��ͼ�� CO��H2��Ϊ��Ҫ��ȼ�Ϻͻ���ԭ�ϣ�����ʮ�ֹ㷺��Ӧ�ã�
CO��H2��Ϊ��Ҫ��ȼ�Ϻͻ���ԭ�ϣ�����ʮ�ֹ㷺��Ӧ�ã�