��Ŀ����
����Ŀ����ѧ�Ľ����벻��������ͻ�ơ�ԭ�ӹ��˴Ź���X�������䡢���Ӽ���ȼ����ķ�չ��Ӧ�ö��ƽ��˽ṹ���о��������Ԫ��ԭ�ӽṹ�������ӽṹ������ṹ�������ṹ�о��ȡ�
(1)����Ԫ��Crԭ�ӵĻ�̬�����Ų�ʽΪ______��Cr�ĺ�������ɼ���̬ԾǨ����̬ʱ�����Ĺ�����______ (����ա����䡱)���ס�
(2)���f����[C(NH2)3+]������������(CH3SO3-)�γɳ����Ӿ��壬��ֲ��ṹ��ͼ��ʾ��

����ɸþ����Ԫ���е縺��������______������S���ӻ�������_______��
��Ԫ��C��N��S�ļ��⻯����ˮ�е��ܽ�ȴ�С�����˳��Ϊ______��ԭ����______��
(3)�ٽ�����ͨ��CuSO4��Һ�У�������ɫ����������ͨ���������������ܽ⣬�õ���ɫ����Һ���ù��������ı仯��[Cu(H2O)4]2+��Cu(OH)2��[Cu(NH3)4]2+��[Cu(H2O)4]2+��[Cu(NH3)4]2+�й�ͬ���еĻ�ѧ��������_________��
����֪:AlF63+����Һ�п��ȶ����ڣ�CaF2������ˮ���������ں�Al3+����Һ�У�ԭ����_____(�����ӷ���ʽ��ʾ)��
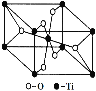
(4)�����о���Աͨ�����ӻ�ѧ����Ԥ�Ⲣ�ϳ��˻�����Na2He����X������������侧���ṹ��ͼ��ʾ��

�پ�����Na�ѻ��γ�___________(����״)�ռ�ṹ��Heռ��һ���϶����һ����e-ռ�ݡ���֪Na2He���岻�ܵ��磬������__________��
����֪�����߳�Ϊanm��������Na�İ뾶Ϊbnm����He�İ뾶Ϊ_____nm(�г�����ʽ����)��
���𰸡� [Ar]3d54s1(��1s22s22p63s23p63d54s1) ���� O sp3 CH4 <H2S< NH3 CH4Ϊ�Ǽ��Է��ӣ�NH3��H2S��Ϊ���Է��ӣ���NH3����ˮ�γ���� ���Լ�����λ�� 3CaF2+Al3+=3Ca2++AlF63- ������ û�������ƶ������Ӻ͵��� ![]()
����������1������Ԫ��Crԭ�ӵĻ�̬�����Ų�ʽΪ��[Ar]3d54s1(��1s22s22p63s23p63d54s1)��Cr�ĺ�������ɼ���̬ԾǨ����̬ʱ�����Ĺ����Ƿ�����ף��ʴ�Ϊ��[Ar]3d54s1(��1s22s22p63s23p63d54s1)�����䣻
��2���ٵ縺������Ԫ��ΪOԪ�أ�S�γ���4���Ҽ������ݼ۲���ӶԻ���ģ���ж�SΪsp3�ӻ���
��ˮΪ�����ܼ���CH4Ϊ�Ǽ��Է��ӣ�NH3��H2S��Ϊ���Է��ӣ���NH3����ˮ�γ�������ٽ����������ԭ�������ж�C��N��S�ļ��⻯����ˮ�е��ܽ�ȴ�С����Ϊ��CH4 <H2S< NH3���ʴ�Ϊ��CH4 <H2S< NH3��CH4Ϊ�Ǽ��Է��ӣ�NH3��H2S��Ϊ���Է��ӣ���NH3����ˮ�γ������
��3���� ˮ���ӻ��������ж����ڼ��Լ�,Cuԭ�Ӻ�ˮ���ӻ�������֮�������λ��,����[Cu(H2O)6]2+��[Cu(NH3)4]2+�ж�������λ�������Լ����ʴ�Ϊ����λ���ͼ��Լ���
��CaF2������ˮ���������ں�Al3+����Һ�У���Ϊ����Һ��F-��Al3+���γɺ��ѵ����������AlF63��ʹCaF2���ܽ�ƽ�����ƣ��䷴Ӧ�����ӷ���ʽΪ��3CaF2+Al3+=3Ca2++AlF63-��
��4���پ�������ͼ�кڵ���Na��8��Na�γɵ�����ṹ�������壬Na2He��û�������ƶ������Ӻ͵��ӣ����Բ��ܵ��磬�ʴ�Ϊ�������壻û�������ƶ������Ӻ͵��ӡ�
���ɾ����Ľṹ���Է�����֪��Na��He֮��ľ���Ϊ������Խ��ߵ�1/4����Խ��߳�![]() ��Na��Heֱ�ӽӴ�����He�İ뾶Ϊ
��Na��Heֱ�ӽӴ�����He�İ뾶Ϊ![]() ��
��

����Ŀ��һ��������,ͨ�����з�Ӧ�����Ʊ������մɵ�ԭ��Mg0:MgSO4(s)+CO(g) ![]() MgO(s)+CO2(g)+SO2(g)��H>0 �÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ�������
MgO(s)+CO2(g)+SO2(g)��H>0 �÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ�������

ѡ�� | x | y |
A | ѹǿ | CO2��CO�����ʵ���֮�� |
B | �¶� | �����ڻ��������ܶ� |
C | MgSO4������(�������) | CO��ת���� |
D | SO2��Ũ�� | ƽ�ⳣ��K |
A. A B. B C. C D. D