��Ŀ����
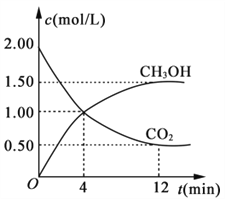
����Ŀ������CO��H2�ڴ����������ºϳɼ״��������ķ�Ӧ���£�CO(g)+2H2(g)![]() CH3OH(g)�������һ�����ܱ������а����ʵ���֮��1��2����CO��H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ������˵����ȷ����
CH3OH(g)�������һ�����ܱ������а����ʵ���֮��1��2����CO��H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ������˵����ȷ����

A. �÷�Ӧ����H��0����p1��p2
B. ��Ӧ���ʣ�����(״̬A)������(״̬B)
C. ��C��ʱ��COת����Ϊ75%
D. �ں��º�ѹ���������ܱ������г��벻ͬ����CH3OH����ƽ��ʱCH3OH���������Ҳ��ͬ
���𰸡�C
�����������������A����ͼ��֪�������¶ȣ�CH3OH�����������С��ƽ�������ƶ�����÷�Ӧ����H��0��300��ʱ������ѹǿ��ƽ�������ƶ���CH3OH�����������������p1��p2����A����B��B���Ӧ���¶Ⱥ�ѹǿ������A�㣬�¶����ߡ�����ѹǿ��ʹ�÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬���������״̬A����������״̬B������B����C�������ܱ�����������1molCO��2molH2��CO��ת����Ϊx����
CO��g��+2H2��g��![]() CH3OH��g��
CH3OH��g��
��ʼ 1 2 0
�仯 x 2x x
���� 1-x 2-2x x
��C��ʱ��CH3OH���������=![]() =0.5�����x=0.75����C��ȷ��D���ɵ�Чƽ���֪���ں��º�ѹ���������ܱ������г��벻ͬ����CH3OH����ƽ��ʱCH3OH�������������ͬ����D����ѡC��
=0.5�����x=0.75����C��ȷ��D���ɵ�Чƽ���֪���ں��º�ѹ���������ܱ������г��벻ͬ����CH3OH����ƽ��ʱCH3OH�������������ͬ����D����ѡC��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣�������ŷֱ����ijһԪ����ش��������⡣
���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | �� | �� |
(1)��-���У�����õĽ���Ԫ���� ______(дԪ�ط��ţ���ͬ)������õ�Ԫ���� ______ �������뵼����ϵ�Ԫ���� ______ ��ijԪ�ص���̬�⻯����������������ˮ�����ֱ�ӻ�������һ���Σ���Ԫ����______��
(2)�ܡ��ݵļ����ӣ���뾶������� ______ (д���ӷ���)��
(3)�ࡢ�����̬�⻯����ȶ����� ______ (�ѧʽ)��
(4)Ԫ�صķǽ����ԣ��� ______ ������![]() ������
������![]() ��)��
��)��
(5)��-�������������ˮ�����У�������ǿ���� ______ (�ѧʽ)��������ǿ���� _________ (�ѧʽ)�� �����������������Ӧ��ˮ���ﷴӦ�����ӷ���ʽΪ ______________________________��
����Ŀ��I������ƽ�ⳣ���Ǻ���������ʵ���̶ȵ�������֪���±����ݣ�25 �棩��
��ѧʽ | ����ƽ�ⳣ�� |
HCN | K��4.9��10��10 |
CH3COOH | K��1.8��10��5 |
H2CO3 | K1��4.4��10��7��K2��4.7��10��11 |
��1��25 ��ʱ����Ũ�ȵ�������Һ��A��NaCN��Һ��B��Na2CO3��Һ��C��CH3COONa��Һ����pH�ɴ�С��˳��Ϊ______(��д���)
��2��25��ʱ����NaCN��Һ��ͨ������CO2����������Ӧ�����ӷ���ʽΪ__________��
��3������Ũ��Ϊ0.02 mol/L��HCN��0.01mol/L NaOH�������Ϻ��c(Na+)>c(CN-)�����й�ϵ��ȷ����____�����ţ���
A��c(H+)>c(OH-) B��c(H+)<c(OH-)
C��c(H+)+c(HCN) = c(OH-) D��c(HCN)+ c(CN-)=0.01mol/L
��4����֪NaHCO3��Һ�ʼ��ԣ�ԭ����____�������ӷ���ʽ��ʾ������д������Һ�и�����Ũ�ȵĴ�С_____________������غ����ʽ_________��
II����Ҫ����գ�
��1���������Ȼ�����Һʱ��Ϊ�˷�ֹ����ˮ�⣬���Լ���������____________��
��2��Ũ��Al2(SO4)3��Һ��Ũ��С�մ�(NaHCO3)��Һ��Ͽ���������������ӷ�Ӧ����ʽ��ʾ����ԭ��_______��
��3����25����pH��12��NaOH��ҺaL��pH=1��HCl��Һb L��ϣ������û��ҺΪ���ԣ���a:b=_____��(��Һ����仯���Բ���)��