��Ŀ����
X��Y��Z��ԭ���������������ͬһ������Ԫ�ء�XΪ����Ԫ�أ�X�ĵ���������ˮ�������ҷ�Ӧ��Y�������������Ǻ�����Ӳ�����2����X��Y���γɻ�����X2Y��Z����Ϊ����һ�Ե��ӶԵ�˫ԭ�ӷ��ӡ�����˵������ȷ����
- A.Y2���Ļ�ԭ�Խ�Z��ǿ
- B.Y���⻯����X2Y������ѧ����������ͬ
- C.YO2��Z2������ΪƯ��������Ư�����õ�ԭ����ͬ
- D.��ʯīΪ�缫���XZ��ˮ��Һ�����������������̪�ɹ۲쵽��Һ�Ժ�ɫ
B

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
X��Y��Z��ԭ�������������������ڲ�ͬ���ڵ����ֳ���������Ԫ�أ�����Ԫ���֮������γ�1��1�͵Ļ�����M��N��R��������M��NΪ���壬RΪҺ�壮M��N����ˮ���ܲ������壬���Ҳ�������������Ի��ϣ�����˵����ȷ���ǣ�������
| A��R���м��Թ��ۼ��ͷǼ��Թ��ۼ� | B��Z�ǵؿ��к�������Ԫ�� | C�����ֻ����ﶼ�ǹ��ۻ����� | D��M��NͶ��ˮ��������Һ��������ͬ |
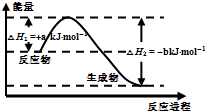 W��X��Y��Z��ԭ������������������ֶ�����Ԫ�أ���֪��W��һ��ԭ�ӵ�ԭ�Ӻ���û�����ӣ�Y��X���ڣ�Y��ZҲ���ڣ�X��Y��Z����Ԫ��ԭ�ӵ�����������֮��Ϊ3����������Ԫ��Z�ڵؿ��к�����ߣ��Իش����и��⣺
W��X��Y��Z��ԭ������������������ֶ�����Ԫ�أ���֪��W��һ��ԭ�ӵ�ԭ�Ӻ���û�����ӣ�Y��X���ڣ�Y��ZҲ���ڣ�X��Y��Z����Ԫ��ԭ�ӵ�����������֮��Ϊ3����������Ԫ��Z�ڵؿ��к�����ߣ��Իش����и��⣺

