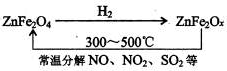
��Ŀ����
���������������Ҫ�����������������ʡ�������ij��ѧѧϰС��Զ����������ʵ���֤ʵ�顣
(1)ʵ����Ʒ����Һ©����_________�������������ƣ����Թܡ���֧�Թܡ��������ܡ��齺�ܣ��������ƾ��塢����( 75%~90%)��NaOHϡ��Һ��NaOHŨ��Һ������KMnO4��Һ��Na2Sϡ��Һ����̪�Լ���
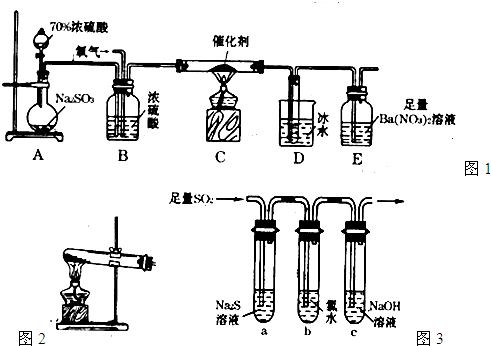
(2)ʵ��װ��
(1)ʵ����Ʒ����Һ©����_________�������������ƣ����Թܡ���֧�Թܡ��������ܡ��齺�ܣ��������ƾ��塢����( 75%~90%)��NaOHϡ��Һ��NaOHŨ��Һ������KMnO4��Һ��Na2Sϡ��Һ����̪�Լ���
(2)ʵ��װ��

װ��ͼ�з�Һ©���ڵ��Լ���_________��д��ѧʽ����
(3)ʵ�鲽��
�ٰ���ͼʾװ�����Ӻ�ʵ�����������װ�������ԡ���鷽����______________________��
���ھ�֧�Թܢ��зֱ����2 mL NaOHϡ��Һ����Һ�е��з�̪�Լ�����2 mL����KMnO4�� Һ��2 mL Na2Sϡ��Һ�����Т��е�ʵ��������_____________����������˵��SO2����_________�ԣ�����������ͨ��SO2��д��������Ӧ�Ļ�ѧ����ʽ____________________��
(4)�������֧�Թܢ��ڵ�����KMnO4��Һ��Ϊ��ˮ����ô��֧�Թܢ��з�����Ӧ��������_________��������Ӧ�����ӷ���ʽ��______________________��
(5)�Թ�V�м������NaOHŨ��Һ����������__________��
(3)ʵ�鲽��
�ٰ���ͼʾװ�����Ӻ�ʵ�����������װ�������ԡ���鷽����______________________��
���ھ�֧�Թܢ��зֱ����2 mL NaOHϡ��Һ����Һ�е��з�̪�Լ�����2 mL����KMnO4�� Һ��2 mL Na2Sϡ��Һ�����Т��е�ʵ��������_____________����������˵��SO2����_________�ԣ�����������ͨ��SO2��д��������Ӧ�Ļ�ѧ����ʽ____________________��
(4)�������֧�Թܢ��ڵ�����KMnO4��Һ��Ϊ��ˮ����ô��֧�Թܢ��з�����Ӧ��������_________��������Ӧ�����ӷ���ʽ��______________________��
(5)�Թ�V�м������NaOHŨ��Һ����������__________��
(1)Բ����ƿ
(2)H2SO4
(3)�ٹر�ֹˮ�У���Һ©���Ļ��������Һ©���л���ע��ˮ������ʼʱ��Һ©���е�ˮ��������Բ����ƿ�У����ŷ�Һ©���м���ˮ�������ӣ�©���е�ˮ���������µΣ���˵������װ������������
�ں�ɫ����ȥ����ԭ��5SO2+2Na2S+2H2O=3S��+4NaHSO3
(4)��ˮ��dz����ɫ��ȥ��Cl2 +SO2+2H2O=4H++2Cl-+SO42-
(5)����SO2β������ֹ�����Ⱦ�Ͷ���
(2)H2SO4
(3)�ٹر�ֹˮ�У���Һ©���Ļ��������Һ©���л���ע��ˮ������ʼʱ��Һ©���е�ˮ��������Բ����ƿ�У����ŷ�Һ©���м���ˮ�������ӣ�©���е�ˮ���������µΣ���˵������װ������������
�ں�ɫ����ȥ����ԭ��5SO2+2Na2S+2H2O=3S��+4NaHSO3
(4)��ˮ��dz����ɫ��ȥ��Cl2 +SO2+2H2O=4H++2Cl-+SO42-
(5)����SO2β������ֹ�����Ⱦ�Ͷ���

��ϰ��ϵ�д�
�����Ŀ