��Ŀ����
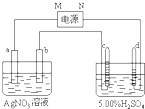
��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ���b�缫��֣�����c��d�缫����������������Ϲ��ռ���336mL����״̬�����壮�ش�
��1��ֱ����Դ�У�MΪ______����
��2��Pt�缫�����ɵ�������______��������Ϊ______g��
��3����Դ����ĵ��ӣ������ʵ�����缫b��c��d�ֱ����ɵ����ʵ����ʵ���֮��Ϊ��2��______��______��______��
��4��AgNO3��Һ��Ũ��______�����������С�����䡱����ͬ����AgNO3��Һ��pH______��H2SO4��Һ��Ũ��______��H2SO4��Һ��pH______��
��1��ֱ����Դ�У�MΪ______����
��2��Pt�缫�����ɵ�������______��������Ϊ______g��
��3����Դ����ĵ��ӣ������ʵ�����缫b��c��d�ֱ����ɵ����ʵ����ʵ���֮��Ϊ��2��______��______��______��
��4��AgNO3��Һ��Ũ��______�����������С�����䡱����ͬ����AgNO3��Һ��pH______��H2SO4��Һ��Ũ��______��H2SO4��Һ��pH______��

��1����������֪���缫a��b��û���������ɣ���a��������bΪ����������M��ԭ����������ʴ�Ϊ������
��2�����ϡ���ᷴӦʱ����ͨ��һ��ʱ���c��d�������ռ���336mL���壨��״����������������2H++2e-�TH2������������4OH--4e-�TO2��+2H2O��ʵ���ϵ�����ˮ����ط�ӦʽΪ��2H2O
2H2��+O2�������ݷ���ʽ֪���������������ռ�������
���Ի�������������������224mL������2H++2e-�TH2��֪��ת�Ƶ��ӵ����ʵ�����0.02mol������������ת�Ƶ�������ȣ���Ƴ�������������������Ϊ
Ag++e-=Ag
1mol 108g
0.02mol 2.16g
�ʴ�Ϊ������2.16g��
��3��b�缫�ϵĵ缫��ӦʽΪ��Ag++e-=Ag
C���缫�ϵĵ缫��ӦʽΪ��4OH--4e-=2H2O+O2��
d���缫�ϵĵ缫��ӦʽΪ��2H++2e-=H2��
����ת�Ƶ��ӵ����ʵ�����2mol����������2mol����������0.5mol����������1mol������
���Ե��ӵ����ʵ�����缫b��c��d�ֱ����ɵ����ʵ����ʵ���֮��Ϊ��2��2��0.5��1��
�ʴ�Ϊ��2��0.5��1��
��4���ڲ��缫�϶���ʱ�������ϵĵ缫��ӦʽΪAg-e-=Ag+�������ϵĵ缫��ӦʽΪAg++e-=Ag��������������Һ��Ũ�Ȳ��䣬��Һ��pH���䣻���ϡ������Һʱ����������2H++2e-�TH2������������4OH--4e-�TO2��+2H2O��ʵ���ϵ�����ˮ����Һ�е����ʲ��䣬�ܼ����٣�����ϡ�����Ũ��������Һ��pHֵ��С��
�ʴ�Ϊ�����䣻���䣻����С��
��2�����ϡ���ᷴӦʱ����ͨ��һ��ʱ���c��d�������ռ���336mL���壨��״����������������2H++2e-�TH2������������4OH--4e-�TO2��+2H2O��ʵ���ϵ�����ˮ����ط�ӦʽΪ��2H2O
| ||
| 2 |
| 3 |
Ag++e-=Ag
1mol 108g
0.02mol 2.16g
�ʴ�Ϊ������2.16g��
��3��b�缫�ϵĵ缫��ӦʽΪ��Ag++e-=Ag
C���缫�ϵĵ缫��ӦʽΪ��4OH--4e-=2H2O+O2��
d���缫�ϵĵ缫��ӦʽΪ��2H++2e-=H2��
����ת�Ƶ��ӵ����ʵ�����2mol����������2mol����������0.5mol����������1mol������
���Ե��ӵ����ʵ�����缫b��c��d�ֱ����ɵ����ʵ����ʵ���֮��Ϊ��2��2��0.5��1��
�ʴ�Ϊ��2��0.5��1��
��4���ڲ��缫�϶���ʱ�������ϵĵ缫��ӦʽΪAg-e-=Ag+�������ϵĵ缫��ӦʽΪAg++e-=Ag��������������Һ��Ũ�Ȳ��䣬��Һ��pH���䣻���ϡ������Һʱ����������2H++2e-�TH2������������4OH--4e-�TO2��+2H2O��ʵ���ϵ�����ˮ����Һ�е����ʲ��䣬�ܼ����٣�����ϡ�����Ũ��������Һ��pHֵ��С��
�ʴ�Ϊ�����䣻���䣻����С��

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ
 ��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ���ֻ��c��d�����Ϲ��ռ���336mL����״̬�����壮�ش�
��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ���ֻ��c��d�����Ϲ��ռ���336mL����״̬�����壮�ش�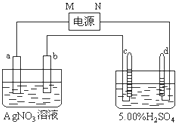 ��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ���b�缫��֣�����c��d�缫����������������Ϲ��ռ���336mL����״̬�����壮�ش�
��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ���b�缫��֣�����c��d�缫����������������Ϲ��ռ���336mL����״̬�����壮�ش�
