��Ŀ����
 ijͬѧ�õ������壨CuSO4?5H2O ������0.40mol/L��CuSO4��Һ240mL���ش���������
ijͬѧ�õ������壨CuSO4?5H2O ������0.40mol/L��CuSO4��Һ240mL���ش���������
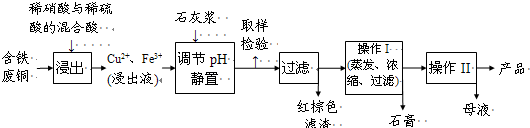
��1������������������ƽ��ҩ�ס��ձ�����ͷ�ιܣ�����Ҫ��Щ��������������ɸ�ʵ�飿��д��______��
��2����д����ʵ���Ҫ��ʵ�鲽�裺
�ټ��� �ڳ�������______g�����ܽ⡡��ת�ơ���ϴ�Ӳ�ת�Ƣ��� ��ҡ��
��3����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����______��
��4����ͬѧ�ڶ��ݺӸǵ�תҡ�ȣ�����Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ����ͬѧ��õ���ҺŨ��______ ���ƫ�ߡ�����ƫ�͡���ȷ������
��5��һƿ���ƺõ�ϡCuSO4��Һ����ʱ���������ǩ����Ϊ����������ʢ��CuSO4��Һ��˵˵���ʵ����鷽����
| ������� | ��ѡ�Լ� | ��Ӧ�����ӷ���ʽ |
| Cu2+ | ______ | ______ |
| SO42- | ______ | ______ |
�⣺��1��ʵ����û��240mL����ƿ��ѡ��250mL����ƿ��
��Һ����һ�㲽���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬���ò��������裬��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�
������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ�
�ʻ���Ҫ�IJ��������У�250mL����ƿ����������
�ʴ�Ϊ��250mL����ƿ����������
��2��ʵ����û��240mL����ƿ��ѡ��250mL����ƿ��������ͭ���������Ϊm=0.25L��0.40mol?L-1��250g/mol=25.0g��
�ʴ�Ϊ��25.0��
��3��ת����Һʱ����ֱ���㵹��Ӧ�ò�����������
�ʴ�Ϊ��ת����Һʱ����ֱ���㵹��Ӧ�ò�����������
��4���Ӹǵ�תҡ�ȣ�������Һ������ƿ����ƿ��֮�䣬�ٵμ�����ˮ���̶��ߣ���Һ��Ũ��ƫ��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��5������Һ��������������������Һ������ɫ��״�������ɣ�˵������ͭ���ӣ���Ӧ���ӷ���ʽΪCu2++2OH-=Cu��OH��2����
ȡ������Һ������ϡ���ᣬBaCl2��Һ���а�ɫ�������ɣ�˵��������������ӣ���Ӧ���ӷ���ʽΪ��Ba2++SO42-=BaSO4����
�ʴ�Ϊ��
��
��������1����Һ���Ƶ�һ����������У����㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������������Һ��ʵ���������ѡ�������������
��2��ʵ����û��240mL����ƿ��ѡ��250mL����ƿ������n=cv���������ͭ�����ʵ���������ͭ�����غ��֪����ͭ������ͭ��������ʵ�����ȣ��ٸ���m=nM������������ͭ�����������
��3��ת����Һʱ����ֱ���㵹��Ӧ�ò�����������
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c= �����жϣ�
�����жϣ�
��5������ͭ������Ӧ����������ͭ��ɫ��������ͭ���ӣ�
����������뱵�������ɲ�����������ᱵ��ɫ����������������ӣ�
���������⿼��һ�����ʵ���Ũ����Һ�����ơ����Ӽ���ȣ��ѶȲ���ע�����c= ������Һ����ԭ������������
������Һ����ԭ������������
��Һ����һ�㲽���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬���ò��������裬��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�
������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ�
�ʻ���Ҫ�IJ��������У�250mL����ƿ����������
�ʴ�Ϊ��250mL����ƿ����������
��2��ʵ����û��240mL����ƿ��ѡ��250mL����ƿ��������ͭ���������Ϊm=0.25L��0.40mol?L-1��250g/mol=25.0g��
�ʴ�Ϊ��25.0��
��3��ת����Һʱ����ֱ���㵹��Ӧ�ò�����������
�ʴ�Ϊ��ת����Һʱ����ֱ���㵹��Ӧ�ò�����������
��4���Ӹǵ�תҡ�ȣ�������Һ������ƿ����ƿ��֮�䣬�ٵμ�����ˮ���̶��ߣ���Һ��Ũ��ƫ��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��5������Һ��������������������Һ������ɫ��״�������ɣ�˵������ͭ���ӣ���Ӧ���ӷ���ʽΪCu2++2OH-=Cu��OH��2����
ȡ������Һ������ϡ���ᣬBaCl2��Һ���а�ɫ�������ɣ�˵��������������ӣ���Ӧ���ӷ���ʽΪ��Ba2++SO42-=BaSO4����
�ʴ�Ϊ��
| Cu2+ | NaOH��Һ | Cu2++2OH-=Cu��OH��2�� |
| SO42- | ϡ���ᣬBaCl2��Һ | Ba2++SO42-=BaSO4�� |
��������1����Һ���Ƶ�һ����������У����㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������������Һ��ʵ���������ѡ�������������
��2��ʵ����û��240mL����ƿ��ѡ��250mL����ƿ������n=cv���������ͭ�����ʵ���������ͭ�����غ��֪����ͭ������ͭ��������ʵ�����ȣ��ٸ���m=nM������������ͭ�����������
��3��ת����Һʱ����ֱ���㵹��Ӧ�ò�����������
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
 �����жϣ�
�����жϣ���5������ͭ������Ӧ����������ͭ��ɫ��������ͭ���ӣ�
����������뱵�������ɲ�����������ᱵ��ɫ����������������ӣ�
���������⿼��һ�����ʵ���Ũ����Һ�����ơ����Ӽ���ȣ��ѶȲ���ע�����c=
 ������Һ����ԭ������������
������Һ����ԭ������������ 
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ