��Ŀ����
�Իش��������⣺
��1����֪24��A��40��Bǡ����ȫ��Ӧ����0��8molC��32��D����C��Ħ������Ϊ ��
��2����ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
�ٸ�Ũ������HCl�����ʵ���Ũ��Ϊ mol/L��
����ʵ��������450mL2��38 mol/L��ϡ���ᣬ���ø�Ũ����________ mL,����ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������______________________(����������)��
��ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________2��38mol/L (����ڡ������ڡ���С�ڡ�����ͬ)��
��1��40g��mol_1 (û��λ��0��)
��2�� ��11��9 ��100 A��C(ȫ�Բŵ÷�)���ձ���������(ȫд�ŵ÷�)�� С��
���������������1�����������غ㶨�ɣ�C������Ϊ��24g+40g-32g=32g����C��Ħ������Ϊ��32g��0��8mol=40g/mol��
��2��������������Ϊ1L�����Ũ����HCl�����ʵ���Ũ��Ϊ��1000��1��19��36��5%��36��5mol?L?1 =11��9mol?L?1��
����Ϊ����ƿ���û��450mL������Ӧѡ��500mL����ƿ��������Ũ��������ΪVL����0��5L��2��38mol?L?1=V��11��9mol?L?1���ɵ�V=0��1L=100mL��ȡŨ�����õ���Ͳ��������Һ�õ�500mL����ƿ������ʱ�õ���ͷ�ιܣ����Բ���ҪA��ƿ��C��Һ©����ϡ��Ũ���ỹ��Ҫ�ձ��Ͳ����������ֲ�������������ʱ���ӿ̶��ߣ���ʹ��Һ����������������Һ��Ũ��С��2��38mol?L?1��
���㣺���⿼�������غ㶨�ɵ�Ӧ�á����ʵ���Ũ�ȵļ��㡢һ�����ʵ���Ũ�ȵ���Һ�����ơ�

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�ijУ��ѧС��ѧ�����С�������Է��������IJⶨ����ʵ�顣�������£����������ݻ�����ȵ���ƿ�ռ����壬�����ռ����������ƿ���������ݼ��±�(�ѻ���ɱ�״���µ���ֵ)��
| ���� | ��ƿ�������������(g) |
| A | 48.4082 |
| B | 48.4082 |
| C | 48.4082 |
| D | 48.4342 |
| E | 48.8762 |
��֪��״���£���ƿ���ݻ�Ϊ0.293 L����ƿ�Ϳ�����������Ϊ48.4212 g��������ƽ����Է�������Ϊ29��A��B��C��D��E����ѧ���������塣
(1)�������������У��ܹ�ʹƷ����Һ��ɫ����(д��ѧʽ)________��
(2)D����Է���������________��
(3)�ڱ�״���£�11.2 L D�����к��й��õ��ӶԵ���ĿΪ________��
(4)A��B��C���ܵĻ�ѧʽ��________��
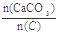 =2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
=2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ�� Ϊ��ֵ��
Ϊ��ֵ��

