��Ŀ����
ͼ��A��Ϊ��25mL 0.1 mol��L-1 NaOH��Һ����εμ�0.2 mol��L-1 CH3COOH��Һ�����У���pH�Ƹ��ٲ����ҺpH�ı仯���ߡ���ش�
��1�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ�� ����ѡ����ĸ��
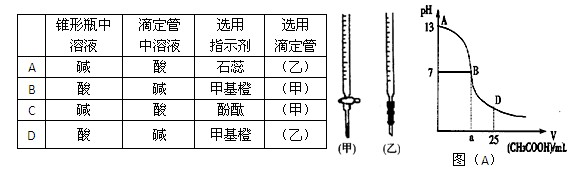
ͼ��A����B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ�� ��ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ� ���䣨����ȷ�����ʲ��𣩡�
AB�����ڣ�����A��B���㣩����Һ����������Ũ�ȵĴ�С��ϵ������_____��______��
��D��ʱ����Һ��c��CH3COO����+c��CH3COOH�� 2c��Na+�������������������������
��11�֣�
C������2�֣�
��1�֣�����AB������2�֣�
c(Na��)>c(OH��)>c(CH3OO��)>c(H��)��c(Na��)>c(CH3OO��)>c(OH��)>c(H��) ����2�֣�
(4)������2�֣�

��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д� ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
�����Ŀ


