��Ŀ����
�Ͼ�����ӵ�ص�����������������Ҫ����LiCoO2������Al��Fe�ȣ���ͨ������ʵ�鷽�������ܡ�ﮣ�

��1���������ܽ�����У�S2O32����������SO42����LiCoO2���ܽ�����з�Ӧ�����ӷ���ʽΪ______��
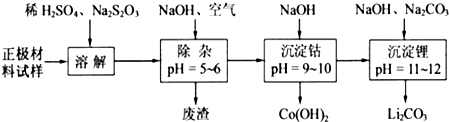
��2������ʱͨ�������Ŀ��______�����õķ����ɷ�Ϊ______���ӷ����л��Al2O3�IJ���������ͼ����ʾ�����ű�ʾ������Լ��������ʾ���õ������ʣ�����I��Ҫ�IJ���������______��������з�Ӧ�����ӷ���ʽ��______��
��3����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ�����ͼ�ң�
a����Li2CO3�������������۵�����Һ��LiOH��Һ������Һ������������ѡ����Ĥ�������ö��Ե缫��⣮
b��������LiOH��Һ�м������NH4HCO3��Һ�����ˡ���ɵøߴ�Li2CO3��
��a�У������ĵ缫��Ӧʽ��______��
��b�У�����Li2CO3��Ӧ�Ļ�ѧ����ʽ��______��

��1���������ܽ�����У�S2O32����������SO42����LiCoO2���ܽ�����з�Ӧ�����ӷ���ʽΪ______��
| ������ | ��ʼ����pH | ������ȫpH |
| Al��OH��3 | 3.0 | 5.2 |
| Fe��OH��3 | 1.5 | 2.8 |
| Fe��OH��2 | 7.6 | 9.7 |
��3����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ�����ͼ�ң�
a����Li2CO3�������������۵�����Һ��LiOH��Һ������Һ������������ѡ����Ĥ�������ö��Ե缫��⣮
b��������LiOH��Һ�м������NH4HCO3��Һ�����ˡ���ɵøߴ�Li2CO3��
��a�У������ĵ缫��Ӧʽ��______��
��b�У�����Li2CO3��Ӧ�Ļ�ѧ����ʽ��______��
��1����������֪�����������£�S2O32����������SO42������LiCoO2����������Co2+�����Է��������ӷ�Ӧ����ʽΪ��S2O32��+8LiCoO2+22H+�T2SO42��+8Li++8Co2++11H2O��
�ʴ�Ϊ��S2O32��+8LiCoO2+22H+�T2SO42��+8Li++8Co2++11H2O��
��2���������Ӳ��ȶ����ױ������������������ӣ����Գ���ʱͨ�������Ŀ���ǽ����������������������ӣ��ٽ�������ת��Ϊ���������������Ӷ���ȥ�������ӣ����ݱ���֪��pHΪ5-6ʱ�������Ӻ���������ȫת��Ϊ���������Է����ijɷ���Fe��OH��3��Al��OH��3������������Һ�ķ����ǹ��ˣ����˹�������Ҫ�IJ����������ձ���©����������������I�У������е������������Ʒ�Ӧ����ƫ�����ƣ�����II�У���ƫ��������Һ��ͨ������Ķ�����̼��ʹƫ������ת��Ϊ������������Ӧ���ӷ���ʽΪ2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32����AlO2-+CO2+2H2O�TAl��OH��3��+HCO3-��
�ʴ�Ϊ����Fe2+������Fe3+�� Fe��OH��3��Al��OH��3���ձ���©������������2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32����AlO2-+CO2+2H2O�TAl��OH��3��+HCO3����
��3����a�У������������ӷŵ������������缫��ӦʽΪ��2H++2e-�TH2����2H2O+2e-�TH2+2OH-��
�ʴ�Ϊ��2H++2e-�TH2����2H2O+2e-�TH2+2OH-��
��b�У�������﮺�̼����立�Ӧ����̼��﮺�̼��泥���Ӧ����ʽΪ2LiOH+2NH4HCO3�TLi2CO3��+��NH4��2CO3��
�ʴ�Ϊ��2LiOH+2NH4HCO3�TLi2CO3��+��NH4��2CO3��
�ʴ�Ϊ��S2O32��+8LiCoO2+22H+�T2SO42��+8Li++8Co2++11H2O��
��2���������Ӳ��ȶ����ױ������������������ӣ����Գ���ʱͨ�������Ŀ���ǽ����������������������ӣ��ٽ�������ת��Ϊ���������������Ӷ���ȥ�������ӣ����ݱ���֪��pHΪ5-6ʱ�������Ӻ���������ȫת��Ϊ���������Է����ijɷ���Fe��OH��3��Al��OH��3������������Һ�ķ����ǹ��ˣ����˹�������Ҫ�IJ����������ձ���©����������������I�У������е������������Ʒ�Ӧ����ƫ�����ƣ�����II�У���ƫ��������Һ��ͨ������Ķ�����̼��ʹƫ������ת��Ϊ������������Ӧ���ӷ���ʽΪ2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32����AlO2-+CO2+2H2O�TAl��OH��3��+HCO3-��
�ʴ�Ϊ����Fe2+������Fe3+�� Fe��OH��3��Al��OH��3���ձ���©������������2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32����AlO2-+CO2+2H2O�TAl��OH��3��+HCO3����
��3����a�У������������ӷŵ������������缫��ӦʽΪ��2H++2e-�TH2����2H2O+2e-�TH2+2OH-��
�ʴ�Ϊ��2H++2e-�TH2����2H2O+2e-�TH2+2OH-��
��b�У�������﮺�̼����立�Ӧ����̼��﮺�̼��泥���Ӧ����ʽΪ2LiOH+2NH4HCO3�TLi2CO3��+��NH4��2CO3��
�ʴ�Ϊ��2LiOH+2NH4HCO3�TLi2CO3��+��NH4��2CO3��

��ϰ��ϵ�д�
�����Ŀ




 ������ӵ���ѱ��㷺������Яʽ��Դ�����ܵ���Դ�ѷ����������һ����չ��
������ӵ���ѱ��㷺������Яʽ��Դ�����ܵ���Դ�ѷ����������һ����չ�� ��һ��DZ�ڵ�����ӵ���������ϣ�������ͨ��
��һ��DZ�ڵ�����ӵ���������ϣ�������ͨ�� ��
�� ��
�� ��Һ������������Ӧ�����ó�����80����ո�����³��Ͷ��Ƶá�
��Һ������������Ӧ�����ó�����80����ո�����³��Ͷ��Ƶá�
 �������ܽ�����У�
�������ܽ�����У� ��������
�������� ��
�� �ڿ����м���ʱ��������������¶ȵı仯
�ڿ����м���ʱ��������������¶ȵı仯