��Ŀ����
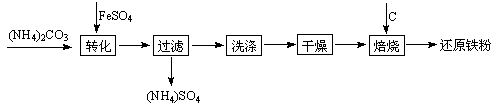
��ԭ�����Ƿ�ĩұ��ҵ����Ҫԭ�ϣ����������Ѱĸ���Ʒ�̷��Ʊ���ԭ���۵Ĺ�ҵ�������£�
��1�����������Ҫ��Ϊ����ȥ����ˮ�ͽᾧˮ�������л�������FeCO3?nH2O����������ΪFeOOH���仯ѧ����ʽΪ______��
��2��ȡ������FeCO3��Ʒ12.49g������600�棬������Ϊ8.00g�������������յõ�Fe 6.16g����600�����Ŀ������Ϊ______������FeCO3��Ʒ��FeCO3��FeOOH������______��
��3������28.12g��ԭ���ۣ�������FexC�����������м��ȣ��õ���״���µ�CO2224mL������ͬ�����Ļ�ԭ�������������ᷴӦ���õ���״���µ�H2 10.752L������FexC�Ļ�ѧʽΪ______�������軹ԭ���۽����������ʣ�
�⣺��1�����������Ҫ��Ϊ����ȥ����ˮ�ͽᾧˮ�������л�������FeCO3?nH2O����������ΪFeOOH������������ԭ��Ӧ�ĵ����غ��ԭ���غ㣬д����ѧ����ʽΪ��4FeCO3?nH2O+O2=4FeOOH+4CO2��+��4n-2��H2O���ʴ�Ϊ��4FeCO3?nH2O+O2=4FeOOH+4CO2��+��4n-2��H2O��
��2��ȡ������FeCO3��Ʒ12.49g������600�棬������Ϊ8.00g�������������յõ�Fe 6.16g��������ƷΪFeCO3��FeOOH������Ԫ�ػ��ϼۿ�֪��Ԫ�ػ��ϼ�Ϊ���ۺ����ۣ����ȱ���600�棬������Ϊ8.00g���Ƿֽ����������������������̼��ˮ��������Ԫ�ػ��ϼ��жϣ��������к����������Ӻ������ӣ�������ΪFeO��Fe2O3��FeO��Fe3O4��FeO��Fe2O3��Fe3O4��
����Ʒ��FeCO3�����ʵ���Ϊx��FeOOH���ʵ���Ϊy��
������Ԫ���غ�õ���x+y=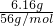 ��116x+89y=12.49
��116x+89y=12.49
����õ�x=0.1mol��y=0.01mol��
�õ�����Ϊ��FeCO3��11.6 g��FeOOH��0.89 g��
�ʴ�Ϊ��FeO��Fe2O3��FeO��Fe3O4��FeO��Fe2O3��Fe3O4��FeCO3��11.6 g��FeOOH��0.89 g��
��3������28.12g��ԭ���ۣ�������FexC�����������м��ȣ��õ���״���µ�CO2224mL�����ʵ���Ϊ0.01mol����FexC���ʵ���Ϊ0.01mol������ͬ�����Ļ�ԭ�������������ᷴӦ���õ���״���µ�H2 10.752L�����ʵ���Ϊ0.48mol�����������ʵ������ݷ�Ӧ����ʽ���㣺Fe+H2SO4=FeSO4+H2������������=0.48mol��56g/mol=26.88g������Ԫ���غ��֪FexC�� ̼Ԫ�����ʵ���Ϊ0.01mol��FexC������=28.12g-26.88g=1.24g�����ʵ���Ϊ0.01mol����Ħ������=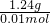 =124g/mol��
=124g/mol��
���ݻ�ѧʽFexC����õ�x=2�����Ի�ѧʽΪFe2C���ʴ�Ϊ��Fe2C��
��������1��FeCO3?nH2O����������ΪFeOOH������������ԭ��Ӧ�����غ��ԭ���غ���ƽ��д��
��2��������Ʒ�к���FeCO3��FeOOH����ע��Ԫ�ػ��ϼۿ�֪Ϊ������Ԫ�غͶ�����Ԫ�أ����ȷֽ�������Ԫ�ص�������������к��ж�������������������������Ϊ���������������������������������жϣ�������Ԫ���غ��������ϵ���㣻
��3��������Ԫ���غ��ϻ�ԭ���ۺ�����FexC�����������м��ȣ��������ɶ�����̼������̼Ԫ�����ʵ����õ�FexC���ʵ��������ݻ�ԭ�����ۺ����ᷴӦ���ɵ��������ʵ�������õ������ʵ��������ݻ�ԭ�����ۻ������������õ�FexC����������ϼ���������ʵ�������xֵ��
���������⿼���������仯�������ʵķ����жϺͷ�Ӧ����ļ����жϣ�Ԫ���غ㡢Ԫ�ػ��ϼ��ǽ���ؼ�����Ŀ�Ѷ��еȣ�
��2��ȡ������FeCO3��Ʒ12.49g������600�棬������Ϊ8.00g�������������յõ�Fe 6.16g��������ƷΪFeCO3��FeOOH������Ԫ�ػ��ϼۿ�֪��Ԫ�ػ��ϼ�Ϊ���ۺ����ۣ����ȱ���600�棬������Ϊ8.00g���Ƿֽ����������������������̼��ˮ��������Ԫ�ػ��ϼ��жϣ��������к����������Ӻ������ӣ�������ΪFeO��Fe2O3��FeO��Fe3O4��FeO��Fe2O3��Fe3O4��
����Ʒ��FeCO3�����ʵ���Ϊx��FeOOH���ʵ���Ϊy��
������Ԫ���غ�õ���x+y=
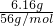 ��116x+89y=12.49
��116x+89y=12.49����õ�x=0.1mol��y=0.01mol��
�õ�����Ϊ��FeCO3��11.6 g��FeOOH��0.89 g��
�ʴ�Ϊ��FeO��Fe2O3��FeO��Fe3O4��FeO��Fe2O3��Fe3O4��FeCO3��11.6 g��FeOOH��0.89 g��
��3������28.12g��ԭ���ۣ�������FexC�����������м��ȣ��õ���״���µ�CO2224mL�����ʵ���Ϊ0.01mol����FexC���ʵ���Ϊ0.01mol������ͬ�����Ļ�ԭ�������������ᷴӦ���õ���״���µ�H2 10.752L�����ʵ���Ϊ0.48mol�����������ʵ������ݷ�Ӧ����ʽ���㣺Fe+H2SO4=FeSO4+H2������������=0.48mol��56g/mol=26.88g������Ԫ���غ��֪FexC�� ̼Ԫ�����ʵ���Ϊ0.01mol��FexC������=28.12g-26.88g=1.24g�����ʵ���Ϊ0.01mol����Ħ������=
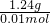 =124g/mol��
=124g/mol�����ݻ�ѧʽFexC����õ�x=2�����Ի�ѧʽΪFe2C���ʴ�Ϊ��Fe2C��
��������1��FeCO3?nH2O����������ΪFeOOH������������ԭ��Ӧ�����غ��ԭ���غ���ƽ��д��
��2��������Ʒ�к���FeCO3��FeOOH����ע��Ԫ�ػ��ϼۿ�֪Ϊ������Ԫ�غͶ�����Ԫ�أ����ȷֽ�������Ԫ�ص�������������к��ж�������������������������Ϊ���������������������������������жϣ�������Ԫ���غ��������ϵ���㣻
��3��������Ԫ���غ��ϻ�ԭ���ۺ�����FexC�����������м��ȣ��������ɶ�����̼������̼Ԫ�����ʵ����õ�FexC���ʵ��������ݻ�ԭ�����ۺ����ᷴӦ���ɵ��������ʵ�������õ������ʵ��������ݻ�ԭ�����ۻ������������õ�FexC����������ϼ���������ʵ�������xֵ��
���������⿼���������仯�������ʵķ����жϺͷ�Ӧ����ļ����жϣ�Ԫ���غ㡢Ԫ�ػ��ϼ��ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ

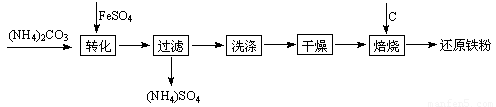
 �ĸ���Ʒ�̷��Ʊ���ԭ���۵Ĺ�ҵ�������£�
�ĸ���Ʒ�̷��Ʊ���ԭ���۵Ĺ�ҵ�������£�