��Ŀ����
A��B��D�����ֳ����Ķ�����Ԫ�أ����ǵ�ԭ������������BԪ��ԭ������������3������DԪ��ԭ��������������һ�룬AԪ��ԭ��������������B��һ�����ס��ҡ�����������ۺ���������Σ����к�BԪ�أ����к�DԪ�أ����к�AԪ�أ��ס�����ˮ��Һ���н�ǿ�ļ��ԣ��ҵ�ˮ��ҺpHС��7����Ϊһ�����壬��Ϊ����ɫ���塣�ס��ҡ����������졢����������֮������Ӧ��ϵ����ͼ��(ͼ����ȥ������ת��ʱ�IJ���������)

�ش��������⣺
��1��д��A��B��D����Ԫ�ص�Ԫ������_____________��_____________��_____________��
��2��д����ĵ���ʽ_________________________��
��3���õ���ʽ��ʾ������AD2�Ĺ���____________________________��
��4��������Һ������������Һ�ֱ�ͼס�����ҺҲ�ܷ�����ѧ��Ӧ���������ﲻ���ѺͶ���д��������������Һ�ֱ�ͼ���Һ������Һ��Ӧʱ�����ӷ�Ӧ����ʽ��
��+��______________________________��
��+��______________________________��
��1��д��A��B��D����Ԫ�ص�Ԫ������_____________��_____________��_____________��
��2��д����ĵ���ʽ_________________________��
��3���õ���ʽ��ʾ������AD2�Ĺ���____________________________��
��4��������Һ������������Һ�ֱ�ͼס�����ҺҲ�ܷ�����ѧ��Ӧ���������ﲻ���ѺͶ���д��������������Һ�ֱ�ͼ���Һ������Һ��Ӧʱ�����ӷ�Ӧ����ʽ��
��+��______________________________��
��+��______________________________��
��1��̼��������
��2��
��3��
��4��H++AlO2-+H2O==Al(OH)3��
H++CO32-==HCO3-
��2��

��3��

��4��H++AlO2-+H2O==Al(OH)3��
H++CO32-==HCO3-

��ϰ��ϵ�д�
�����Ŀ
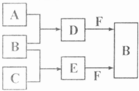 A��B��C�����ֳ���������Ԫ�صĵ��ʣ�������DΪ��ɫҺ�壬E��һ�ֳ������������壮��ת����ϵ��ͼ����Ӧ�����Ͳ��ֲ�����ȥ�����Իش�
A��B��C�����ֳ���������Ԫ�صĵ��ʣ�������DΪ��ɫҺ�壬E��һ�ֳ������������壮��ת����ϵ��ͼ����Ӧ�����Ͳ��ֲ�����ȥ�����Իش�

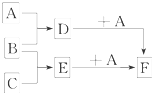 ��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧʱ�ɹ۲쵽��ɫ���棬����K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��
��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧʱ�ɹ۲쵽��ɫ���棬����K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��
