��Ŀ����
16��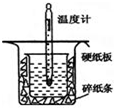 ��1���� 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺
��1���� 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺���ձ�����������ĭ���ϵ������Ǽ�С����ɢʧ��
�������ձ��ϸ�Ϊ�DZ����壬��õķ�Ӧ����ֵ��ƫС���ƫ����ƫС������Ӱ�족��
����ͨ���ⶨ�������������Ϊ1.42kJ����д�÷�Ӧ���Ȼ�ѧ����ʽ��HCl��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-56.8kJ•mol-1��
��2������֪��CH4��g��+H2O��g���TCO��g��+3H2��g����H=+206.2kJ•mol-1
CH4��g��+CO2��g���T2CO��g��+2H2��g����H=+247.4kJ•mol-1
���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH4��g����H2O��g����Ӧ����CO2��g����H2��g�����Ȼ�ѧ����ʽΪ��CH4��g��+2H2O��g���TCO2��g��+4H2��g����H=+165.0kJ•mol-1��
������̬��̬ԭ���γ�1mol��ѧ���ͷŵ���������м��ܣ���֪�������м������ݣ���N2��g��+3H2��g��?2NH3��g����H=-93kJ•mol-1��
| ��ѧ�� | H-H | N-H | N��N |
| ����kJ•mol-1 | 436 | 391 | 945 |
���� ��1�����к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
�������ձ��ϸ�Ϊ�DZ����壬��ʹһ��������ɢʧ��
�۸����Ȼ�ѧ����ʽ�������Լ���д�������ش�
��2���ٸ��ݸ�˹���ɼ��㷴Ӧ�ȣ��ݴ���д���Ȼ�ѧ��Ӧ����ʽ��
�ڸ��ݷ�Ӧ�ȵ��ڷ�Ӧ����ܼ���-��������ܼ������㣮
��� �⣺��1�����ձ�����������ĭ���ϵ������ǣ����£�����ȡ������������ʧ�����ʴ�Ϊ�����£�����ȡ������������ʧ����
�������ձ��ϸ�Ϊ�DZ����壬��ʹһ��������ɢʧ����õķ�Ӧ����ֵƫС���ʴ�Ϊ��ƫС��
��50mL 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ��Ӧ����0.025molˮ������������Ϊ1.42kJ��������1molˮ����56.8kJ����HCl��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-56.8kJ•mol-1��
�ʴ�Ϊ��HCl��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-56.8kJ•mol-1��
��2����a��CH4��g��+H2O��g���TCO��g��+3H2��g����H=+206.2kJ•mol-1
b��CH4��g��+CO2��g���T2CO��g��+2H2��g����H=+247.4kJ•mol-1
�ɸ�˹���ɿ�֪��a��2-b�ɵã�CH4��g��+2H2O��g���TCO2��g��+4H2��g�� H=+165.0 kJ•mol-1
�ʴ�Ϊ��CH4��g��+2H2O��g���TCO2��g��+4H2��g����H=+165.0 kJ•mol-1��
��N2��g��+3H2��g��???2NH3��g����H=945kJ•mol-1+436kJ•mol-1��3-391kJ•mol-1��6=-93kJ•mol-1=a kJ•mol-1�����a=-93���ʴ�Ϊ��-93��
���� ������Ҫ�����к��Ȳⶨ����˹���ɵ�Ӧ�ã���Ӧ�ȵļ��㣬ע�ⷴӦ�ȵ��ڷ�Ӧ����ܼ���-��������ܼ��ܣ�

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�| A�� | Fe3+�������ԣ�ʵ���ҳ���KSCN��Һ����Fe3+ | |
| B�� | SO2�н�ǿ�Ļ�ԭ�ԣ�SO2��ʹ����KMnO4��Һ��ɫ | |
| C�� | Mg�л�ԭ�ԣ����MgCl2������Һ���Ʊ�Mg | |
| D�� | ŨH2SO4����ˮ�ԣ�ŨH2SO4��ʹ����̿�� |
| A�� | NaCl��Cl2 | B�� | NaHCO3������ | C�� | Ba��OH��2��HCl | D�� | H2SO4������ |
| A�� | ÿ����«�������к���6������� | |
| B�� | ��«����̼���⡢������Ԫ����� | |
| C�� | ��«������Է�������Ϊ228 | |
| D�� | ��«����̼���⡢������Ԫ�ص�������Ϊ14��12��3 |
| A�� | �ֹ�$��_{����}^{����}$ SiCl4 $��_{����}^{����}$Si | |
| B�� | Mg��OH��2 $\stackrel{����}{��}$ MgCl2��aq��$\stackrel{���}{��}$ Mg | |
| C�� | Fe2O3 $\stackrel{����}{��}$ FeCl3��aq��$\stackrel{����}{��}$ ��ˮFeCl3 | |
| D�� | AgNO3��aq��$\stackrel{NH_{3}•H_{2}O}{��}$[Ag��NH3��2]OH��aq��$��_{����}^{����}$ Ag |
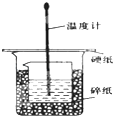 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺��1����ʵ���л�ȱ��һ���������������ǻ��β�����������ڴ�С�ձ��������ĭ���ϵ������DZ��¸��ȣ���ֹ����ɢʧ��
��2����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50mL������¼��ԭʼ���ݣ���������
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2��/�� | �²� ��t2-t1��/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�ȣ�2�������Hƫ���ƫ����ƫС�����䡱����
��4�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����������¶ȼƲⶨNaOH��Һ�¶ȵIJ��裬���˲������裬���õ��к��ȡ�H��ƫ���ƫ����ƫС�����䡱����
