��Ŀ����
��֪��2H2(g)��O2(g)==2H2O(l) ��H����571.6 kJ��mol��1
2CH3OH(l)��3O2(g)==2CO2(g)��4H2O(l) ��H����1452 kJ��mol��1
H��(aq)��OH��(aq)==H2O(l) ��H����57.3 kJ��mol��1
����˵����ȷ���ǣ� ��
A�� H2(g)��ȼ����Ϊ571.6 kJ��mol��1
H2(g)��ȼ����Ϊ571.6 kJ��mol��1
B��ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų���������
C��1/2H2SO4(aq)��1/2Ba(OH)2(aq)== 1/2BaSO4(s)��H2O(l) ��H����57.3 kJ��mol��1
D��3H2(g)��CO2(g)=CH3OH(l)��H2O(l) ��H����135.9 kJ��mol��1
��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
����Һ����ʾ���Ĺ�����ʹ�õĻ�ѧ��ϴ��NF3��һ���������壬�䴢��������������CO2�ĵ�12000��20000�����ڴ����е������ɳ���740��֮�ã������Ǽ��ֻ�ѧ���ļ��ܣ�
��ѧ�� | N��N | F-F | N-F |
����/KJ��mol-1 | 941.7 | 154.8 | 283.0 |
����˵������ȷ����
A������N2(g)��2N(g)�ų�����
B������N(g)+3F(g)��NF3(g)�ų�����
C����ӦN2(g)+3F2(g)��2NF3(g)�ġ�H��0
D��NF3�������������û�л�ѧ���Ķ��������ɣ��Կ��ܷ�����ѧ��Ӧ


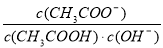 ����
���� 2SO3(g)���Ʊ��������Ҫ��Ӧ������������ȷ����( )
2SO3(g)���Ʊ��������Ҫ��Ӧ������������ȷ����( )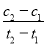
 ����ʹ�����¶ȡ��ܶ���ͬ������ѹǿ��p����С�Ĺ�ϵ����
����ʹ�����¶ȡ��ܶ���ͬ������ѹǿ��p����С�Ĺ�ϵ���� I2(g) + H2(g)��ƽ�������˵���϶���ȷ���ǣ���
I2(g) + H2(g)��ƽ�������˵���϶���ȷ���ǣ���