��Ŀ����
����A��B��C��D��E��F���ֶ���������Ԫ�أ����ǵ�ԭ��������������Dԭ�Ӻ���������ԭ�ӹ������2��δ�ɶԵ��ӣ�D��A�γɵĻ�������ӹ�����V�͡�BԪ�����γɻ�������������Ԫ�أ�A��B������������֮����C��������������ȣ�F��ͬ���ڵ�һ��������С��Ԫ�ء�
��1��д��CԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽ ��B��C��D����Ԫ�ص縺���ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ�� ��
��2��E��A���γɷ���ʽΪEA�Ļ�����ӽ�EA�е��EA��������Է�����������ֵҪ��������Ӽ���ֵ��ԭ���� ��C��D�ֱ���A�γ�������У��ȶ��Խϸߵ��� ���ѧʽ�����ӣ��÷�������A+��ϳ�����ʱ��ACA���ļ��ǻ� �����������䡱��С����
��3��C��F�γɵĻ�����FC3�У�C3�Ŀռ乹��Ϊ ��C3������ԭ�Ӳ��� �ӻ�������FC3ʱ���������������ɫ�����ԭ���Ͻ��Ͳ����������ԭ�� ��
��4����ͼΪBԪ�ص�ij�ֵ��ʾ���ľ�������þ�����Bԭ�ӵĿռ�ռ����Ϊ ����r��ʾB��ԭ�Ӱ뾶���ô�r�ı���ʽ��ʾ�����ؼ�

��1��2s22p3 O��N��C
��2��HF���Ӽ�ͨ����������γ�(HF)n���ӣ�ʹ��Է����������� H2O
��3��ֱ���� �ڼ��������£����ܼ�����ԾǨ�����ܼ������ȶ��������ɸ��ܼ�ԾǨ�ص��ܼ��������ԹⲨ��ʽ�ͷţ�ʹ�������������ɫ
��4�� ��
��
��������
���������Dԭ�Ӻ���������ԭ�ӹ������2��δ�ɶԵ��ӣ���֪D��p��������Ų�Ϊ��p2����p4D��A�γɵĻ�������ӹ�����V�ͣ���D��p��������Ų�Ϊ��p4��DΪ��Ԫ�أ�AΪ��Ԫ�ء�BԪ�����γɻ�������������Ԫ�أ���BΪ̼Ԫ�أ�CΪ��Ԫ�ء�F��ͬ���ڵ�һ��������С��Ԫ�أ���FΪ��Ԫ�أ�EΪ��Ԫ�ء�
��1��CΪ��Ԫ�أ��۵����Ų�Ϊ��2s22p3��ͬ����Ԫ�ش������ҵ縺�����������Ե縺���ɴ�СΪ��O��N��C��
��2��E��A���γɷ���ʽΪEA�Ļ�����ΪHF��HF���Ӽ���ں�ǿ��������������γɶ���壨HF��n
������Է�������Ҫ����HF��C��D�ֱ���A�γ������ΪNH3��H2O���ǽ�����O��N�������⻯����ȶ���H2O��NH3��H2O��H+�γ�H3O+��H2O�������Թ¶Ե��ӣ���H3O+ֻ��һ�Թ¶Ե��ӣ��¶Ե�����Ŀ���٣��Թ��ۼ����ų���Ҳ��С�����H-O-H���DZ��
��3��C��F�γɵĻ�����FC3ΪNaN3��N3-��CO2Ϊ�ȵ����壬���ĵ�ԭ�Ӳ�ȡsp�ӻ������N3-�ռ乹��Ϊֱ���͡�����NaN3ʱ����ԭ�ӵ��ܼ�����ԾǨ�����ܼ��γɼ���̬������̬���ȶ��������ִӼ���̬Ǩ�ػ�̬���Ӹ��ܼ�ԾǨ�����ܼ����ų������������ԹⲨ����ʽ���ڣ�ʹ��������������ɫ��
��4����ԭ�ӱ�ʾ���ǽ��ʯ�ľ�����ͨ������һ�����ʯ��������̼ԭ����Ϊ�� ����̼ԭ�ӵİ뾶Ϊr����̼ԭ����ռ�����ΪV1=
����̼ԭ�ӵİ뾶Ϊr����̼ԭ����ռ�����ΪV1= ���辧���ı߳�Ϊa�����ݽ��ʯ�����Ľṹ����
���辧���ı߳�Ϊa�����ݽ��ʯ�����Ľṹ���� ����
���� ���������V=��
���������V=�� ��3����þ�����Bԭ�ӵĿռ�ռ����Ϊ��
��3����þ�����Bԭ�ӵĿռ�ռ����Ϊ�� ��100%
��100%
���㣺���⿼�����ʽṹ�����ʡ����忼��Ԫ�����ڱ�������Ԫ�ؼ۵����Ų����ɡ��縺�Թ��ɡ��ǽ����⻯����ȶ��ԡ���������ӵ��ӻ�������ۺͿռ乹�͡�����ṹ��ؼ��㡣

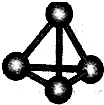 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






