��Ŀ����
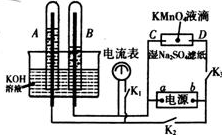
(12��)ij��Ǧ�����ؾ������ۡ������������������ص㣬��ʹ�õĵ�����ǿ����Եļ�����Ǧ����صĹ���ԭ����

�ŷŵ�ʱ�������ĵ缫��ӦʽΪ �����ʱ��Pb�缫Ӧ����������ӵ�Դ�� (�������������)��
�ƹ�ҵ��PbO2���Ʊ�KClO4�Ĺ�ҵ�������£�

��д��NaClO3��PbO2��Ӧ�����ӷ���ʽ�� ��
�ڹ�ҵ�Ͽ���������Һ����KNO3������Ӧ�Ʊ�KClO4��ԭ���� ��
��PbO2�����¶������ֽ⣬��ȡ23.9gPbO2�������ȷֽ�����и����ʵ��������¶ȵı仯����ͼ��ʾ��
����ij�¶��²��ʣ����������Ϊ22.94g������¶���PbO2�ֽ����ù����������Ϊ ��д��ѧʽ���������ʵ���֮��Ϊ ��

�ŷŵ�ʱ�������ĵ缫��ӦʽΪ �����ʱ��Pb�缫Ӧ����������ӵ�Դ�� (�������������)��
�ƹ�ҵ��PbO2���Ʊ�KClO4�Ĺ�ҵ�������£�

��д��NaClO3��PbO2��Ӧ�����ӷ���ʽ�� ��
�ڹ�ҵ�Ͽ���������Һ����KNO3������Ӧ�Ʊ�KClO4��ԭ���� ��
��PbO2�����¶������ֽ⣬��ȡ23.9gPbO2�������ȷֽ�����и����ʵ��������¶ȵı仯����ͼ��ʾ��
����ij�¶��²��ʣ����������Ϊ22.94g������¶���PbO2�ֽ����ù����������Ϊ ��д��ѧʽ���������ʵ���֮��Ϊ ��

��4H++PbO2+2e����Pb2++2H2O ���� �Ƣ�PbO2+ClO3��+2H+=Pb2++ClO4��+H2O
��KClO4���ܽ�Ƚ�С ��Pb2O3��Pb3O4 1��1
��KClO4���ܽ�Ƚ�С ��Pb2O3��Pb3O4 1��1
��1���ŵ��൱����ԭ��أ������õ����ӣ����Ե缫��ӦʽΪ4H++PbO2+2e����Pb2++2H2O������൱�ڵ��أ�zauԭ�����Pb�Ǹ�����ʧȥ���ӡ������ٳ��ʱӦ�ú͵�Դ�ĸ������������������õ����ӡ�
��2���ٶ�����Ǧ���������ԣ��ܰ��������������ɸ������ƣ�����ʽΪ
PbO2+ClO3��+2H+=Pb2++ClO4��+H2O��
������KClO4���ܽ�Ƚ�С���ᾧ������
��3��������Ǧ��0.1mol��������ԭ����0.02mol����A�㣬�������0.8g����ʣ����ԭ�ӵ����ʵ�����0.15mol��ʣ���ʱǦ����ԭ�ӵĸ���֮����2�U3��ʣ��A���Ӧ��������Pb2O3��ͬ���ɵó�B���Ӧ������Pb3O4������ʣ�������������A��B֮�䣬���Թ�������Pb2O3��Pb3O4��ɵ��������ߵ����ʵ����ֱ���x��y����2x+3y��0.1��3x+4y��0.2�� ��0.14�����x��y��0.02mol��������1�U1�ġ�
��0.14�����x��y��0.02mol��������1�U1�ġ�
��2���ٶ�����Ǧ���������ԣ��ܰ��������������ɸ������ƣ�����ʽΪ
PbO2+ClO3��+2H+=Pb2++ClO4��+H2O��
������KClO4���ܽ�Ƚ�С���ᾧ������
��3��������Ǧ��0.1mol��������ԭ����0.02mol����A�㣬�������0.8g����ʣ����ԭ�ӵ����ʵ�����0.15mol��ʣ���ʱǦ����ԭ�ӵĸ���֮����2�U3��ʣ��A���Ӧ��������Pb2O3��ͬ���ɵó�B���Ӧ������Pb3O4������ʣ�������������A��B֮�䣬���Թ�������Pb2O3��Pb3O4��ɵ��������ߵ����ʵ����ֱ���x��y����2x+3y��0.1��3x+4y��0.2��
 ��0.14�����x��y��0.02mol��������1�U1�ġ�
��0.14�����x��y��0.02mol��������1�U1�ġ�
��ϰ��ϵ�д�
�����Ŀ




 ���������й�˵���������(����)
���������й�˵���������(����)