��Ŀ����
ijУ��ѧ��ȤС��Ϊ�ⶨ��������ֵ������ȣ�ʵ������������Լ��Ͳ����������±���ʾ�����������У���Ӧ����ȫ����ʵ����� | �������A�� | ��������ˮ | �����Լ���B�� | ���ˡ�ϴ�ӡ�����ù��壨C�� | ������Һ�õ����壨D�� | ����������� |
�� | BaCl2��NaCl | �ܽ� | ����CO2 | �� | D | A��D |
�� | CaCl2��NaCl | �ܽ� | ��������Na2CO3��Һ | CaCO3 | NaCl | A��C |
�� | Na2SO4��MgSO4 | �ܽ� |
|
|
|
|
�Իش��������⣺
��1��ʵ�����D�ijɷ���____________��������____________����ܡ����ܡ���ͨ������õ�A�и���ֵ������ȡ�
��2��ʵ�����д�����������Դ������____________����ԭ����_____________________��
��3����ʵ��������ƣ�������Ӧ����������ж�Ӧ�Ŀո��ڣ������ܵó�A�и���ֵ�������Ϊ�����ú���ĸ�ı���ʽ��ʾ��_____________________________________________��
��1��BaCl2��NaCl ����
��2��D D�������NaCl������Na2CO3
��3������NaOH��Һ Mg(OH)2 Na2SO4��NaOH A��C��A![]() ����
����![]() �������������𰸣�
�������������𰸣�
��������3���ʣ�Na2SO4��MgSO4�����������ײ�����������壬���������׳��������ij�����ѡ�ù���NaOH��Һ������
Mg(OH)2һ�ֳ��������������Ӧ�����������Ϊ��������A��Mg(OH)2����C��Na2SO4��MgSO4�����ȵı���ʽΪ��A![]() ����
����![]() ��
��



 ��I���ζ��������ǻ�ѧ�������е���Ҫ��������֮һ��
��I���ζ��������ǻ�ѧ�������е���Ҫ��������֮һ��

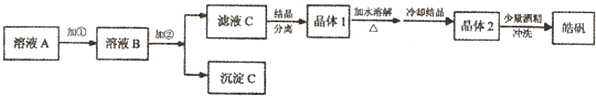
 ��2013?����һģ��ijУ��ѧ��ȤС�����������ʵ��װ�ã�ͼ�в��ּг���������ȥ������ ��ij��̼�Ͻ�������������������̽������Ũ����ķ�Ӧ����ش��������⣺
��2013?����һģ��ijУ��ѧ��ȤС�����������ʵ��װ�ã�ͼ�в��ּг���������ȥ������ ��ij��̼�Ͻ�������������������̽������Ũ����ķ�Ӧ����ش��������⣺