��Ŀ����
��1��H��H�ļ���Ϊ436 kJ/mol������ʾ��������___________________
��2��һ����ѧ��Ӧ�ķ�Ӧ�ȣ��跴Ӧ��������Ϊ��̬���뷴Ӧ����������еļ���֮�������еĹ�ϵ���ɱ������ݼ��������Ȼ�ѧ����ʽ�е���ЧӦ��H2(g)��Cl2(g) = 2HCl(g)����H=____________��
��3�����ۼ��ļ���ǿ���Ի�ѧ��Ӧ�кܴ��Ӱ�졣±����RX��ͬ�������·�������ˮ�ⷴӦʱ
��RX+NaOH �� R-OH+NaX������RF��RCl��RBr��RI��R��ͬ���ķ�Ӧ�����ɴ�С��˳����___________________��
��4����Ԥ��C��Br���ļ��ܷ�Χ��д������ֵ��_______< C��Br����<_________��
��2��-185kJ��mol-1
��3��RF<RCl<RBr<RI
��4��218kJ��mol-1��330kJ��mol-1

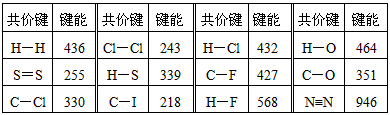
��12�֣����³�ѹ�£�����1mol�����룩������ӻ�ѧ�������յ��������γ�1mol �����룩������ӻ�ѧ�����ų���������Ϊ���ܡ��±���һЩ�������ݣ���λΪKJ��mol-1��
|
��ѧ�� |
���� |
��ѧ�� |
���� |
��ѧ�� |
���� |
|
C��F |
427 |
C��Cl |
330 |
C��I |
218 |
|
H��H |
436 |
S��S |
255 |
H��S |
339 |
�ش��������⣺
(1)�ɱ������ݹ���Ԥ��C��Br���ļ��ܷ�Χ��
_________ <C��Br����<__________
(2)�Ȼ�ѧ����ʽ2H2(g)��S2(g) =2H2S(g)����H= QKJ��mol��1����Q=
(3) ��֪�����Ȼ�ѧ����ʽ��
��֪�����Ȼ�ѧ����ʽ��
O2 (g) = O+2(g) + e�� ��H1= +1175.7 kJ��mol-1
 PtF6(g) + e��= PtF6��(g)
��H2=-771.1 kJ��mol-1
PtF6(g) + e��= PtF6��(g)
��H2=-771.1 kJ��mol-1
O2+PtF6��(s) = O2+(g) + PtF6��(g) ��H3= +482.2 kJ��mol-1
 ��ӦO2(g)
+ (g)
= O2+PtF6�� (s)�ġ�H=___________________ kJ��mol-1��
��ӦO2(g)
+ (g)
= O2+PtF6�� (s)�ġ�H=___________________ kJ��mol-1��
(4)��֪���ʯ��ʯī�ֱ�����������ȫȼ�յ��Ȼ�ѧ����ʽΪ��
C�����ʯ,s��+O2(g) ��CO2(g) ��H=��395.4 kJ•mol��1
C(ʯī,s)+O2(g)��CO2(g) ��H=��393.5 kJ•mol��1
��ʯīת��Ϊ���ʯʱ���Ȼ�ѧ����ʽΪ
�ɴ˿���̼��������ͬ���������и��ȶ���Ϊ ����