��Ŀ����
 ��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ���������
��2���ձ���������ֽ����������
��3�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ
��4�������60mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������
��5������ͬŨ�Ⱥ�����Ĵ��ᣨCH3COOH������HCl��Һ��������ʵ�飬��õ��к��ȵ���ֵ��
��6������ƽ�в�������õ��������£�
| ��ʼ�¶�t1/�� | ��ֹ�¶� T2/�� |
�¶Ȳ� ��t/�� | |||
| HCl | NaOH | ƽ��ֵ | |||
| 1 | 25 | 25 | 27.3 | ||
| 2 | 25 | 25 | 27.4 | ||
| 3 | 25 | 25 | 28.6 | ||
���㣺�к��ȵIJⶨ
ר�⣺ʵ����
��������1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ�����½ϵ�ʱ��Ӧ��ϵ��ɢ�ȣ�
��4����Ӧ�ų����������������Լ�������Ķ����йأ������к��ȵĸ����ʵ�����ش�
��5������������ʵ������ȷ�����
��6�����ݷ�Ӧ����Һ�ı�����cΪ4.18J?��-1?g-1�������ʵ��ܶȾ�Ϊ1g?cm-3�� ���빫ʽ��Q=cm��t2-t1�����к��ȡ�H=-
���㼴�ɣ�
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ�����½ϵ�ʱ��Ӧ��ϵ��ɢ�ȣ�
��4����Ӧ�ų����������������Լ�������Ķ����йأ������к��ȵĸ����ʵ�����ش�
��5������������ʵ������ȷ�����
��6�����ݷ�Ӧ����Һ�ı�����cΪ4.18J?��-1?g-1�������ʵ��ܶȾ�Ϊ1g?cm-3�� ���빫ʽ��Q=cm��t2-t1�����к��ȡ�H=-
| Q |
| n(H2O) |
���
�⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������
�ʴ�Ϊ�����β����������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮��������ֽ���������Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��3�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ����õ��к�����ֵ�����С�����½ϵ�ʱ��Ӧ��ϵ��ɢ�ȱȽϿ죬������ʧ���ƫ�ͣ�
�ʴ�Ϊ��ƫС��ɢ��̫�죻
��4����Ӧ�ų����������������Լ�������Ķ����йأ�������60mL0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ��к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ�
��5������Ϊ���ᣬ�������Ϊ���ȹ��̣����Դ��ᣨCH3COOH������HCl��Һ��Ӧ����Ӧ�ų�������С��57.4kJ���ʴ�Ϊ��ƫС��
��6���������������ϴ���ȥ����t=
=2.35�棬Q=cm��t=2.35���4.18J/��g?�棩��100g=1159J=982.3J=0.9823kJ����H=
=
=-39.3kJ/mol��
�ʴ�Ϊ��39.3kJ/mol��H=-39.3kJ/mol����
�ʴ�Ϊ�����β����������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮��������ֽ���������Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��3�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ����õ��к�����ֵ�����С�����½ϵ�ʱ��Ӧ��ϵ��ɢ�ȱȽϿ죬������ʧ���ƫ�ͣ�
�ʴ�Ϊ��ƫС��ɢ��̫�죻
��4����Ӧ�ų����������������Լ�������Ķ����йأ�������60mL0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ��к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ�
��5������Ϊ���ᣬ�������Ϊ���ȹ��̣����Դ��ᣨCH3COOH������HCl��Һ��Ӧ����Ӧ�ų�������С��57.4kJ���ʴ�Ϊ��ƫС��
��6���������������ϴ���ȥ����t=
| (27.3��-25��)+(27.4��-25��) |
| 2 |
| -Q |
| n(H2O) |
| -0.9823kJ |
| 0.025mol |
�ʴ�Ϊ��39.3kJ/mol��H=-39.3kJ/mol����
���������⿼���Ȼ�ѧ����ʽ�Լ���Ӧ�ȵļ��㣬��Ŀ�Ѷȴ�ע�������к��ȵĸ����Լ��ⶨ��Ӧ�ȵ��������⣮

��ϰ��ϵ�д�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ
 ���ײ��ϵı�������ռ�������ı��������������������������ʵ�ԭ����ij�����������ӷֲ���������þ�������ṹ��ͼ��ʾ���������������ķ���ʽΪ���������� þԭ�ӡ�λ�ڶ���������������ģ���ԭ�ӡ�λ���ڲ� ��
���ײ��ϵı�������ռ�������ı��������������������������ʵ�ԭ����ij�����������ӷֲ���������þ�������ṹ��ͼ��ʾ���������������ķ���ʽΪ���������� þԭ�ӡ�λ�ڶ���������������ģ���ԭ�ӡ�λ���ڲ� ��| A��MgB |
| B��Mg3B6 |
| C��Mg5B12 |
| D��Mg14B6 |
120�桢1.01��105Pa�£�ij����O2�Ļ�����干8L����ȫȼ�պ�õ�ͬ��ͬѹ�µ�����7L��������ǣ�������
| A��C2H6 |
| B��C2H2 |
| C��C2H4 |
| D��C3H4 |
�����ܱ�������A��g��+3B��g��?2C��g������H��0��ƽ����¶Ƚ��ͣ�������������ȷ���ǣ�������
| A�������л�������ƽ����Է���������С |
| B������Ӧ���������淴Ӧ���ʼ�С��ƽ��������Ӧ�����ƶ� |
| C������Ӧ���ʺ��淴Ӧ���ʶ���С��C�İٷֺ������� |
| D����������ܶȵı仯�����������Ϊ�жϷ�Ӧ�Ƿ��ٴδ�ƽ������� |
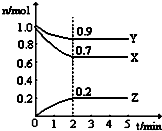 ij�¶�ʱ����һ��2L���ܱ������У�H2��N2��NH3�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ��ش��������⣺
ij�¶�ʱ����һ��2L���ܱ������У�H2��N2��NH3�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ��ش��������⣺ ijͬѧ��NaCl��������100mL 2mol/L��NaCl��Һ����ش��������⣺
ijͬѧ��NaCl��������100mL 2mol/L��NaCl��Һ����ش��������⣺