��Ŀ����
��10�֣�ij��������Ʒ�������������������ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�

������������̣��ش��������⣺
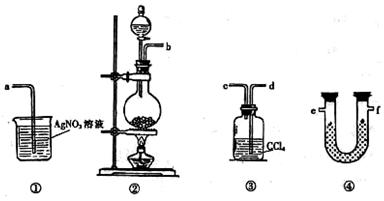
��1������I�г����ձ����������Ҫ�õ��IJ��������� ��
��2������II�õ��������� ��
��3����������������W1 g������������Ⱥ������������W2 g������Ʒ����Ԫ�ص����������� ���г�ԭʼ��ʽ�����軯��
��4��ijͬѧ��Ϊ���ԴӲ�������õ�250.00mL��Һ��ʼ����ƶ���ʵ��ⶨ���������ĺ��������������ͬѧ��ʵ����ơ�
aȡ������Һ25.00mL������ƿ�У�
bȡijŨ�ȵĸ������������Һװ����ʽ�ζ����У���������Һ���¼������
c�ζ�����¼������
d�������ݽ��м���
�ټ�ͬѧ��Ϊa��b�����˵���ָʾ���Ļ��ڣ����ж����ޱ�Ҫ��˵�����ɡ�
��
����ͬѧ��Ϊc��d������һ�����ڣ����ж��Ǻλ��ڣ���˵�����ɡ�
��

������������̣��ش��������⣺
��1������I�г����ձ����������Ҫ�õ��IJ��������� ��
��2������II�õ��������� ��
| A����ʽ�ζ��� | B��50mL��Ͳ | C��10mL��Ͳ | D��25mL��Һ�� |
��4��ijͬѧ��Ϊ���ԴӲ�������õ�250.00mL��Һ��ʼ����ƶ���ʵ��ⶨ���������ĺ��������������ͬѧ��ʵ����ơ�
aȡ������Һ25.00mL������ƿ�У�
bȡijŨ�ȵĸ������������Һװ����ʽ�ζ����У���������Һ���¼������
c�ζ�����¼������
d�������ݽ��м���
�ټ�ͬѧ��Ϊa��b�����˵���ָʾ���Ļ��ڣ����ж����ޱ�Ҫ��˵�����ɡ�
��
����ͬѧ��Ϊc��d������һ�����ڣ����ж��Ǻλ��ڣ���˵�����ɡ�
��
ÿ��2�֣�1�� 250mL����ƿ����ͷ�ι���
��2�� D ��
��3�� 1120��W1-W2��/160a ��
��4���� �ޱ�Ҫ�����������Һ��������ָʾ�������ã�����ɫ�� ��
�� �ظ�����2�Σ����Σ���ʹʵ���õ����ݿ��Բ���(������߾�ȷ��)
��2�� D ��
��3�� 1120��W1-W2��/160a ��
��4���� �ޱ�Ҫ�����������Һ��������ָʾ�������ã�����ɫ�� ��
�� �ظ�����2�Σ����Σ���ʹʵ���õ����ݿ��Բ���(������߾�ȷ��)
��

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ
 mol/L��NaCl��Һ���Խ�ͷ�ιܼ�ˮ����ʱ��Һ���Գ�������ƿ�̶��ߣ�����ҡ�Ⱥ�Һ����͵�ǡ����̶�������
mol/L��NaCl��Һ���Խ�ͷ�ιܼ�ˮ����ʱ��Һ���Գ�������ƿ�̶��ߣ�����ҡ�Ⱥ�Һ����͵�ǡ����̶�������