��Ŀ����
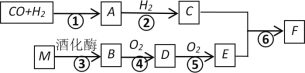
����Ŀ��ij����ʱͬѧ�������ʵ��װ�òⶨˮ����ɡ�
�ش��������⣺
(1)����X������Ϊ____________��
(2)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ______��
(3)��Ӳ�ʲ������й����ɺ�ɫת��Ϊ��ɫʱ����ã���Ӳ�ʲ����ܺ����й������������Ӧ�������1.6g���ڸ���ܵ��������ڷ�Ӧ��������1.82g�����������ݼ���ó�ˮ��H��OԪ�ص�������Ϊ___(�����������)��
(4)��ͬѧ��������Ϊ����װ�ô���ȱ�ݣ�����װ�ý��иĽ����䷽����________(�������ɵ�ˮ��ȫ��D�м�ʯ�����գ�װ���ڵ�ˮ������CO2���Բ���)��
(5)��ͬѧ��������֪��CuO����ԭ�Ĺ����п�����Cn2O(��ɫ)���ɡ�����Ӧ��Ӳ�ʲ������в������庬��Cu2O���Ƿ��Ӱ���ʵ��ⶨ���? _____________(����Ӱ����������Ӱ����)��
���𰸡�����©�� Zn+H2SO4=ZnSO4+H2�� 11��80 ��Dװ�ú���������һ��ʢ�м�ʯ�ҵĸ���� ��Ӱ��
��������
ͨ����Һ©����װ��п������ƿ�м���ϡ���ᣬ���߷�Ӧ����H2��ͨ��Ũ���������H2�ڲ������з�����ӦH2+CuO![]() H2O+Cu������Ӧ��H2+2CuO
H2O+Cu������Ӧ��H2+2CuO![]() H2O+Cu2O��װ��C���ٵ�������������OԪ�ص���������Ӧ������H2O��������еļ�ʯ�����գ�ͨ��Dװ������ ����������H2O��������C��D�����ı�IJ�ֵ����HԪ�ص����������ڿ����к���ˮ�֡�CO2�ᱻ��ʯ�����գ������D���滹��װһ��ʢ�м�ʯ�ҵĸ���ܡ�
H2O+Cu2O��װ��C���ٵ�������������OԪ�ص���������Ӧ������H2O��������еļ�ʯ�����գ�ͨ��Dװ������ ����������H2O��������C��D�����ı�IJ�ֵ����HԪ�ص����������ڿ����к���ˮ�֡�CO2�ᱻ��ʯ�����գ������D���滹��װһ��ʢ�м�ʯ�ҵĸ���ܡ�
(1)���������ṹ��֪������X������Ϊ����©����
(2)��װ��A��Zn��ϡ���ᷢ���û���Ӧ������ZnSO4��H2��������Ӧ�Ļ�ѧ����ʽΪZn+H2SO4=ZnSO4+H2����
(3)��Ӳ�ʲ������й����ɺ�ɫת��Ϊ��ɫʱ����ã���Ӳ�ʲ����ܺ����й������������Ӧ�������1.6g�����ٵ�����������OԪ�ص��������ڸ���ܵ��������ڷ�Ӧ��������1.82g�����ӵ�����ΪH2O�������������к��е�HԪ�ص�����Ϊ1.82g-1.6g=0.22g����ˮ��H��OԪ�ص�������Ϊ0.22g��1.6g=11��80��
(4)�����е�CO2��H2O�ᱻ��ʯ�����գ�����Dװ�����ع��࣬�Ը�װ�ý��иĽ��ķ�������Dװ�ú���������һ��ʢ�м�ʯ�ҵĸ���ܡ�
(5)CuO�е�O��H2��Ӧ����H2O��ÿ����1molOԭ�ӣ���ͬʱ����1molH2O����CuO����ԭ��IJ����أ���������Ӧ��Ӳ�ʲ������в������庬��Cu2O������Ӱ��ʵ��ⶨ�����

 �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�����Ŀ�������仯�����ڹ�ũҵ������������������Ҫ���á����������գ�
�ϳɰ���ҵ�У�N2(g) + 3H2(g) ![]() 2NH3(g) + Q(Q��0)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ�����
2NH3(g) + Q(Q��0)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ�����
t/�� | 200 | 300 | 400 |
K | K1 | K2 | 0.5 |
(1)�ԱȽ�K1��K2�Ĵ�С��K1 ________K2����д����������=������������
(2) 400��ʱ����Ӧ2NH3(g) ![]() N2(g) + 3H2(g)�Ļ�ѧƽ�ⳣ����ֵΪ_____________��
N2(g) + 3H2(g)�Ļ�ѧƽ�ⳣ����ֵΪ_____________��
�����NH3��N2��H2�����ʵ���Ũ�ȷֱ�Ϊ3 mol/L��2 mol/L��1 mol/Lʱ����÷�Ӧ��(N2)(��) ___________��(N2)(��)����д����������=������������
(3)���ܱպ��ݵ������У���������Ϊ�ϳɰ���Ӧ�ﵽƽ������ݵ���____________��
a����(N2)������=3�� (H2)���棩 b�����������ܶȱ��ֲ���
c��������ѹǿ���ֲ��� d��N2��H2��NH3��Ũ��֮��Ϊ1��3��2
(4)��������Ϊ�����������������������ö��ַ�������д������ϳɰ���ҵ��Ϊʵ������������ȡ�Ĵ�ʩ��__________________________��__________________________��
(5)0.1 mol/L��(NH4)2SO4ˮ��Һ�и�����Ũ���ɴ�С��˳����_____________________���ڸ���Һ�м��������������壬��Һ��NH4+��Ũ��_______������������������С������������������ԭ����_________________________________________________________��
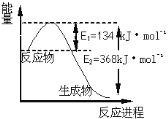
(6)��ͼ��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2�� CO��Ӧ���Ȼ�ѧ����ʽ _____________________________________________��