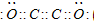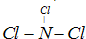��Ŀ����
��14�֣�����A��B��C��D��E���ֶ�����Ԫ�أ���֪���ǵ�ԭ��������������Ԫ��A��ԭ��ֻ��һ�����Ӳ㣻��֪Ԫ��A�ֱܷ���Ԫ��B��C��D�γɵ���������ȵĻ������
��X��Y��Z��Y�д̼�����ζ��Z������ΪҺ̬��Ԫ��E�ĵ��ʵ�ˮ��Һ��Ư���ԡ�
��ش��������⣺
��1��Ԫ��E�ĵ��ʵı�����Һ��ʯ��ʯ��Ӧ��������Һ��Ư������ǿ��ԭ���� ��
��2�� �ڹ�¯ˮ�м���C2A4�����ѹ�¯�������������ԭΪ�ṹ���ܵ����������������㣬C2A4���������ҷ�Ӧ������Ⱦ�����ɣ�д����Ӧ�Ļ�ѧ����ʽ��������ƽ�� ��1 mol C2A4�μӷ�Ӧת�Ƶ��ӵ���ĿΪ ����������Ļ�ѧʽΪ ��
��3��������Y��Ԫ��C����������������ˮ���ﰴ���ʵ�����Ϊ2��1��ȫ��Ӧ���ˮ��Һ�� �ԣ���ᡱ������С�������Һ������Ũ���ɴ�С��˳��Ϊ ��
��4����X��D2 ���ܴ���O2���ĵĹ���������ɵ�ԭ����У������ĵ缫��ӦʽΪ ��
��5��A��B��D����Ԫ�ؿ����γ����ʽΪB2A4D������ζ���л�����л��������������¿���ˮ�⣬���ɺ�̼ԭ������ͬ�����ֳ����л���������л����������ʳƷ��ζ��������������ζ���л�����һ��������ˮ��Ļ�ѧ����ʽ�� ��
1������ˮ�д�������ƽ��Cl2 + H2O  HCl + HClO��1�֣���CaCO3 ����ˮ�е�HCl��Ӧ��ʹ c(H+)��С��1�֣���ʹ����ƽ�������ƶ��� HClOŨ������1�֣�����Ư������ǿ
HCl + HClO��1�֣���CaCO3 ����ˮ�е�HCl��Ӧ��ʹ c(H+)��С��1�֣���ʹ����ƽ�������ƶ��� HClOŨ������1�֣�����Ư������ǿ
��2��6Fe2O3��N2H4��4Fe3O4��N2���� 2H2O��2�֣���4NA��1�֣���N2��1�֣�
��3���1�֣���c (NH4+)> c (NO3��)> c (OH��)> c (H+)��2�֣�
��4��CH4��8e����4O2��= CO2 +2H2O ��2�֣�
��5��CH3COOCH2CH3 + H2O CH3COOH + CH3CH2OH ��2�֣�
CH3COOH + CH3CH2OH ��2�֣�
����

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�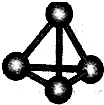 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��