��Ŀ����
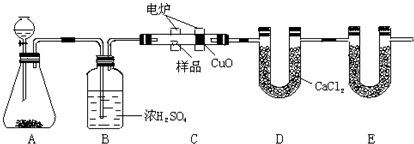
��ѧ�ϳ���ȼ�շ�ȷ���л������ɣ���ͼ��ʾװ������ȼ�շ�ȷ���л��ﻯѧʽ���õ�װ�ã��÷������ڵ�¯����ʱ�ô�������������Ʒ��Ȼ����ݲ��������ȷ���л������ɡ�

�ش��������⣺
(1)Aװ���з�Һ©��ʢ�ŵ������� ��д���йط�Ӧ�Ļ�ѧ����ʽ
(2)Cװ��(ȼ�չ�)��CuO��������
(3)Eװ������ʢ�����ʵ����� ������������
(4)����Bװ��ȥ�����ʵ�������ʲôӰ��?
(5)��ȷ��ȡ1.20g��Ʒ(ֻ��C��H��O����Ԫ���е����ֻ�����)�������ȼ�պ�E����������1.76g��D����������0.72g������л�������ʽΪ
(6)Ҫȷ�����л���Ļ�ѧʽ����Ҫ�ⶨ
(1)H2O2(��˫��ˮ)���� 2H2O2���� ![]() 2H2O+O2��
2H2O+O2��
(2)ȷ���л�������������CO2��H2O
(3)��ʯ�һ��������ơ��� ��������CO2
(4)��ɲ���л����к�����ƫ��
(5)CH2O
(6)�л������Է���������

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ