��Ŀ����
| Cr2O3 |
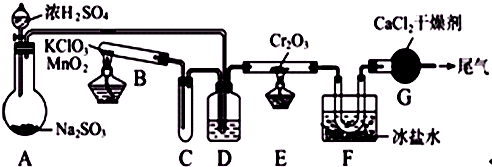
��1��Aװ�����õ��IJ���������
��2��С�Թ�C��������
��3��װ��D�����������ǣ��ٸ���SO2��O2��ʹSO2��O2��Ͼ���
��
��4��ʵ���е�Cr2O3�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȣ��Է��¶ȹ��ߣ���������ԭ����
��5������װ��F�����ȴ��G��β����Ҫ��SO2��O2���������¸������Լ����ʵ����֤β���к���SO2��
02�������õ����Լ��ǣ���NaOH��Һ����Ũ���ᡢ�����Ը��������Һ���ܴ����ǵ�ľ��������������ʵ����Ʒ����ѡ����Ҫ����д�±���
| ʵ�鷽�� | �й�ʵ����������ӷ���ʽ |
| ��G��β���Ⱥ�ֱ�ͨ���Լ���˳���� |
��2��C�еĵ��ܶ��̣ܶ���������������ã�
��3������װ�úͷ�Ӧ�����Լ���Ӧ��ת����������
��4������ӦΪ���ȷ�Ӧ���¶ȹ��߲�����������������ɣ�
��5����֤���ж������ɸ��ݶ�������Ļ�ԭ�������Ը�����أ���֤�����������ɽ����������ȥ��������ô����ǵ�ľ�����飮
��2��ʵ������õ����Ȳ����Լ�����������ˮ��Ҫ��������ʵ����Bװ��ͨ�������Ʊ�O2������ͨ����Һ��Ҫ��ֹ��Ӧ������ѹǿ��������Һ�嵹�����ȵķ�Ӧ�����ڣ�����С�Թ�C�������ǻ��塢��ֹD�е�Һ�嵹����B�У�
�ʴ�Ϊ�����塢��ֹ������
��3��D��ӦΪŨ���ᣬ����������������ã�ͬʱΪ��ַ�Ӧ��ԭ�����ó�֣���ֹ�˷ѣ�����E�Թܵ�����Ҫ��ֻ�ϣ��������ܹ��죬��D����ͨ���۲������������������ٶȵ����ã�
�ʴ�Ϊ��ͨ���۲������������������ٶȣ�
��4����Cr2O3�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȣ��Է��¶ȹ��ߣ���������������ķ�Ӧ����ӦΪ���ȷ�Ӧ���¶ȹ��߲�����������������ɣ���Ӱ������Ļ��ԣ�
�ʴ�Ϊ����Ӧ���ȣ��¶ȹ��߲�����������������ɣ���Ӱ������Ļ��ԣ�
��5����֤���ж������ɸ��ݶ�������Ļ�ԭ�������Ը�����أ���֤�����������ɽ����������ȥ��������ô����ǵ�ľ�����飬����������ͨ�����������Һ�������Ƿ����ɶ�������Ȼ��ͨ������������Һ��ȥ��������Ũ���������ô����ǵ�ľ�����飬��ط�Ӧ�����ӷ���ʽΪ5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��SO2+2OH-=SO32-+H2O��
�ʴ�Ϊ���ۢ٢ڣ��ܣ�5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��SO2+2OH-=SO32-+H2O��

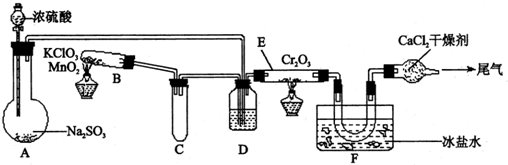
2(08�㽭ʡ������ѧģ��)ʵ�����и���2SO2��O2![]() 2SO3����H=-393.2 kJ?mol-1�������ͼ��ʾʵ��װ�����Ʊ�SO3���塣��ش��������⡣
2SO3����H=-393.2 kJ?mol-1�������ͼ��ʾʵ��װ�����Ʊ�SO3���塣��ش��������⡣
 |
��1��ʵ��ǰ��������еIJ����ǣ���������ƣ�����д������̣�������������
��2����Aװ���м���Na2SO3�����ͬʱ������Ӽ���ˮ��Ȼ���ٵμ�Ũ���ᡣ�Ӽ���ˮ�������� ��
��3��С�Թ�C��������
��4�����ƿD��ʢ���Լ��� ��װ��D������������ ������ ����
�� ��
��5��ʵ���е�Cr2O3�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȣ��Է��¶ȹ��ߣ���������ԭ���� �� ��
��6��װ��F��U�����ռ��������ʵ���ɫ��״̬��
��7��װ��G��������
��8����Gװ�õ�����β������������