��Ŀ����
��8�֣�ijǿ������Һ�п��ܺ��е�������K+��NH4+��Al 3+��AlO2-��SO42-��SiO32-��CO32-��Cl-�е�ij�������ӣ��ֽ�������ʵ�飺
��ȡ��������Һ�������ữ��Ba��NO3��2��Һ���������ɡ�
����ȡ������Һ�������ᣬ�������ǣ�һ��ʱ�䱣��ԭ����ʼ���������������࣬������������������һ�����壬��������������ʧ��
��1��ԭ��Һ�п϶����ڵ������� ��
�϶������ڵ�������_ ��
��2����֪һ������ԭ��Һ�м���5 mL 0.2 mol/L����ʱ����������ȫ��ʧ��������������������Һ�ɵõ�����0.187 g����ԭ��Һ���Ƿ���Cl-��_________________��
��3�����շ�Ӧ˳������д�����з�����Ӧ�����ӷ�Ӧ����ʽ
�� H++OH-=H2O ��
�� �� HCO3-+H+=H2O+CO2
��
��ȡ��������Һ�������ữ��Ba��NO3��2��Һ���������ɡ�
����ȡ������Һ�������ᣬ�������ǣ�һ��ʱ�䱣��ԭ����ʼ���������������࣬������������������һ�����壬��������������ʧ��
��1��ԭ��Һ�п϶����ڵ������� ��
�϶������ڵ�������_ ��
��2����֪һ������ԭ��Һ�м���5 mL 0.2 mol/L����ʱ����������ȫ��ʧ��������������������Һ�ɵõ�����0.187 g����ԭ��Һ���Ƿ���Cl-��_________________��
��3�����շ�Ӧ˳������д�����з�����Ӧ�����ӷ�Ӧ����ʽ
�� H++OH-=H2O ��
�� �� HCO3-+H+=H2O+CO2
��
�� 8�֣�ǰ����ÿ��2�֣���4����ÿ��1�֣�
��1��OH-��AlO2-��CO32-��K+�� NH4+��SO42-��SiO32-��Al 3+��
��2����Cl-��
��3�� AlO2- + H++H2O = Al��OH�� 3 �� CO32-+H+=HCO3- Al��OH�� 3 +3H+=3H2O+Al3+
��1��OH-��AlO2-��CO32-��K+�� NH4+��SO42-��SiO32-��Al 3+��
��2����Cl-��
��3�� AlO2- + H++H2O = Al��OH�� 3 �� CO32-+H+=HCO3- Al��OH�� 3 +3H+=3H2O+Al3+
��1��������Һ��ǿ���ԣ�����һ������OH-����û��NH4+��Al 3+������ʵ��ٿ�֪����Һ��û��SO42-��SiO32-������ʵ��ڿ�֪�������İ�ɫ����������������������CO2������ԭ��Һ��һ�����е�������AlO2-��CO32-��������Һ�ĵ����Կ�֪����K+һ�����У����������Dz���ȷ���ġ�
��2�����ɵ�0.187g��ɫ�������Ȼ��������ʵ�����0.014mol���������������0.001mol������ԭ��Һ�л�һ�����������ӡ�
��3������ԭ��Һ�к���OH-��AlO2-��CO32-���������������к�OH-���ں�AlO2-��Ӧ��������������ɫ������Ȼ���ں�CO32-��Ӧ����HCO3-��Ȼ������CO2������ں�����������Ӧ��
��2�����ɵ�0.187g��ɫ�������Ȼ��������ʵ�����0.014mol���������������0.001mol������ԭ��Һ�л�һ�����������ӡ�
��3������ԭ��Һ�к���OH-��AlO2-��CO32-���������������к�OH-���ں�AlO2-��Ӧ��������������ɫ������Ȼ���ں�CO32-��Ӧ����HCO3-��Ȼ������CO2������ں�����������Ӧ��

��ϰ��ϵ�д�
�����Ŀ
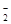 ��Na+��Cl-����Na2O2��HCl��Al2O3�����ʵ���֮����Ϊ�� ��
��Na+��Cl-����Na2O2��HCl��Al2O3�����ʵ���֮����Ϊ�� ��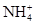 ��Cl��
��Cl�� ��
�� ����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش� ��SO42-��CO32-,���ֻ�������������Ӿ�����ͬ���Ҿ�������ˮ���ֽ����Ƿֱ����0.1 mol��L-1����Һ����������ʵ�飺
��SO42-��CO32-,���ֻ�������������Ӿ�����ͬ���Ҿ�������ˮ���ֽ����Ƿֱ����0.1 mol��L-1����Һ����������ʵ�飺