��Ŀ����
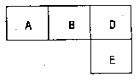 ��M��A��B��D��E���ֶ�����Ԫ�أ���ԭ��������������MԪ�ص�ԭ�Ӱ뾶�����ڱ�����Ԫ������С�ģ�A��B��D��E�ֱ����±��У����ڱ���һ���֣���ͬ�Ŀո���ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37����ش�
��M��A��B��D��E���ֶ�����Ԫ�أ���ԭ��������������MԪ�ص�ԭ�Ӱ뾶�����ڱ�����Ԫ������С�ģ�A��B��D��E�ֱ����±��У����ڱ���һ���֣���ͬ�Ŀո���ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37����ش���1��Ԫ�ط��ţ�A
��2��D��E����Ԫ�طֱ���MԪ���γɵ���������У�����ȶ�����
��3��A��B��D��M����ɶ���18���ӵķ��ӣ����е����ֽṹ�ɷֱ��ʾΪM2B-BM2��AM3-BM2������д��2�־���18���ӵ��л���Ľṹ��ʽ
��4��M��B��D��ԭ�Ӹ�����4��2��3���γɵ����ӻ������ˮ��Һ��
��1��������������֪��AΪC��BΪN��DΪO��EΪS��
�ʴ�Ϊ��C��N��O��S��
��2��D��EԪ�ص�����⻯��ֱ��ǣ�ˮ�����⣬Ԫ�صķǽ�����Խǿ���⻯��Խ�ȶ����ǽ���O��S����H2O���ȶ���ˮ����֮����������������ΪҺ̬��������Ϊ��̬����ˮ�ķе�ϸߣ�
�ʴ�Ϊ��H2O��H2O��
��3��C��N��O��H����ɶ���18���ӷ��ӣ�����18���ӵ��л���һ������̼����Ԫ�أ���ϵ�����Ŀ��֪������̼ԭ����ĿΪ2����CH3CH3����18�����ӣ�һ��Oԭ����һ��CH2���е�������ȣ�������ɵ�CH3OH��
�ʴ�Ϊ��CH3CH3��CH3OH��
��4��H��N��O��ԭ�Ӹ�����4��2��3���γɵ����ӻ�����ΪNH4NO3����Һ��NH4+ˮ�⣬��Һ�����ԣ�ˮ��̶Ȳ���NH4+Ũ��Զ����H+����0.1mol/L�û������ˮ��Һ�и����ӵ�Ũ���ɴ�С�������ǣ�c��NO3-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ���c��NO3-����c��NH4+����c��H+����c��OH-����

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д���M��A��B��D��E���ֶ�����Ԫ�أ���ԭ��������������MԪ�ص�ԭ�Ӱ뾶�����ڱ�����Ԫ������С�ġ�A��B��D��E�ֱ����±��У����ڱ���һ���֣���ͬ�Ŀո���ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37����ش�
��1��Ԫ�ط��ţ�A B D E
��2��D��E����Ԫ�طֱ���MԪ���γɵ���������У�����ȶ����� ���ѧʽ�����е�ϸߵ��� ���ѧʽ����
��3��A��B��D��M����ɶ���18���ӵķ��ӣ����е����ֽṹ�ɷֱ��ʾΪM2B��BM2��
AM3��BM2������д��2�־���18���ӵ��л���Ľṹ��ʽ �� ��
��4��M��B��D��ԭ�Ӹ�����4��2��3���γɵ����ӻ������ˮ��Һ�� �ԡ�0��1mol/L�û������ˮ��Һ�и����ӵ�Ũ���ɴ�С�������� ��
��M��A��B��D��E���ֶ�����Ԫ�أ���ԭ��������������MԪ�ص�ԭ�Ӱ뾶�����ڱ�����Ԫ������С�ġ�A��B��D��E�ֱ����±��У����ڱ���һ���֣���ͬ�Ŀո���ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37����ش�
|
|
|
|
|
|
|
��1��Ԫ�ط��ţ�A B D E
��2��D��E����Ԫ�طֱ���MԪ���γɵ���������У�����ȶ����� ���ѧʽ�����е�ϸߵ��� ���ѧʽ����
��3��A��B��D��M����ɶ���18���ӵķ��ӣ����е����ֽṹ�ɷֱ��ʾΪM2B��BM2��
AM3��BM2������д��2�־���18���ӵ��л���Ľṹ��ʽ �� ��
��4��M��B��D��ԭ�Ӹ�����4��2��3���γɵ����ӻ������ˮ��Һ�� �ԡ�0��1mol/L�û������ˮ��Һ�и����ӵ�Ũ���ɴ�С�������� ��
��M��A��B��D��E���ֶ�����Ԫ�أ���ԭ��������������MԪ�ص�ԭ�Ӱ뾶�����ڱ�����Ԫ������С�ġ�A��B��D��E�ֱ����±��У����ڱ���һ���֣���ͬ�Ŀո���ռ����Ӧ��λ�ã����ǵ�ԭ������֮��Ϊ37����ش�
| | | |
| | | |
��2��D��E����Ԫ�طֱ���MԪ���γɵ���������У�����ȶ����� ���ѧʽ�����е�ϸߵ��� ���ѧʽ����
��3��A��B��D��M����ɶ���18���ӵķ��ӣ����е����ֽṹ�ɷֱ��ʾΪM2B��BM2��
AM3��BM2������д��2�־���18���ӵ��л���Ľṹ��ʽ �� ��
��4��M��B��D��ԭ�Ӹ�����4��2��3���γɵ����ӻ������ˮ��Һ�� �ԡ�0��1mol/L�û������ˮ��Һ�и����ӵ�Ũ���ɴ�С�������� ��


 �����������У���ʹ�˹���˥�ϣ����ڴ��������ºܿ�ת��ΪM2D2��D2�����ʣ���ת�����̿������ӷ���ʽ��ʾΪ��
�����������У���ʹ�˹���˥�ϣ����ڴ��������ºܿ�ת��ΪM2D2��D2�����ʣ���ת�����̿������ӷ���ʽ��ʾΪ��