��Ŀ����
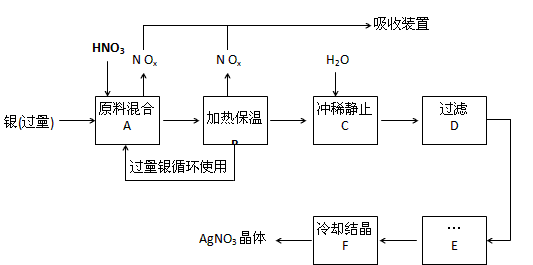
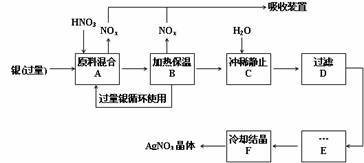
ij�Լ���������������ͭ�������ᣨ������Fe3+����Ӧ��ȡ���������������£�����ͼ����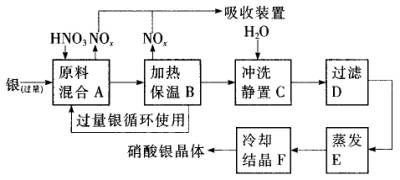
�����������裬���������գ�
��1���ܽ���������Ӧ����________������ϡ������Ũ������ԭ����________��
������a�����ٹ����в���NOx����
������b������ԭ������������
��c����ʡ��������ʵ���
��2������B���ȱ��µ�������________��
������a�������ڼӿ췴Ӧ����
������b��������δ��Ӧ������ӷ�
������c�������������ַ�Ӧ��������Һ��[H+]
��3������C��Ϊ�˳�ȥFe3+��Cu2+�����ʣ���ϴ�����ÿɷ����Ļ�ѧ��Ӧ��________��
������a���û���Ӧ
������b��ˮ�ⷴӦ
������c��������ԭ��Ӧ
���������ij����ﻯѧʽ________��
������
| (1)ϡ��a��c�� ��2��a��c�� ��3��b��Fe(OH)3��Cu(OH)2
|
��ʾ��
| �����������ᷴӦ�Ļ�ѧ����ʽ��
Ag+2HNO3=
AgNO3+NO2 ��֪����ȡͬ����������������ʹ��ϡ���Ũ���ʡ����������������ٲ����к�����NOx�����ǣ�����ϡ���ᷴӦ���еý�������Ҫ���ȣ����ȱ��£������ܼӿ췴Ӧ���ʣ����������������£�Ҳ�����������ַ�Ӧ�ͳ�����ã��Ӷ���������Һ����ȣ��ڼ�ˮϡ��ʱ������Fe3+��Cu2+ˮ�⣬�γ�Fe(OH)3��Cu(OH)2������ͨ�����˳��������õ�������AgNO3.
|

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д���14�֣�ÿ��2�֣�ij�Լ���������������ͭ�������ᣨ��Fe3+����Ӧ��ȡ���������������£�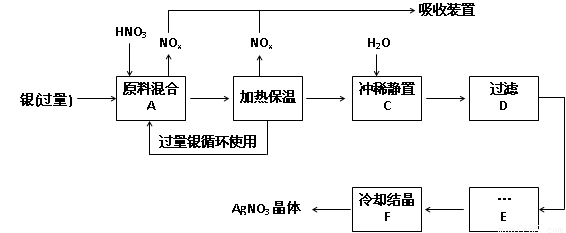
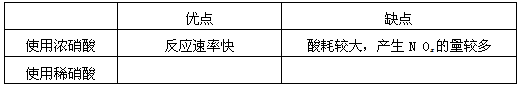
��1����ҵ��һ��ѡ���е�Ũ�ȵ����������Ӧ����ȡ�������������±��ո���ա�
|
|
�ŵ� |
ȱ�� |
|
ʹ��Ũ���� |
��Ӧ���ʿ� |
��Ľϴ���NOx�����϶� |
|
ʹ��ϡ���� |
|
|
��2������B���ȱ��µ������� ��
a�� �����ڼӿ췴Ӧ����
b��������δ��Ӧ������ӷ�
c�������������ַ�Ӧ��������Һ��H+��Ũ��
��3������C��Ϊ�˳�ȥFe3+��Cu2+�����ʣ���ϡʱ����������ԭ���� ��
��4������C�м�ˮ����Ӧ������������������ˮ���Ժ���������ɵIJ���Ӱ���ǣ�
��
��5������E���еIJ����� ��
��6���Ƶõ��������к�����������ͭ��ͨ����ȥ����ͭ�ķ������ڲ���E֮ǰ���������Ƶ�Ag2O��ʹCu2+ת��ΪCu(OH)2��������Ӧ����˳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��12�֣�ij�Լ���������������ͭ�������ᣨ��Fe3+����Ӧ��ȡ���������������£�
��1����ҵ��һ��ѡ���е�Ũ�ȵ����������Ӧ����ȡ�������������±��ո���ա�
| �ŵ� | ȱ�� |
ʹ��Ũ���� | ��Ӧ���ʿ� | ��Ľϴ���NOx�����϶� |
ʹ��ϡ���� |
|
|
��2������B���ȱ��µ������� ��
a�������ڼӿ췴Ӧ����
b��������δ��Ӧ������ӷ�
c�������������ַ�Ӧ��������Һ��H+��Ũ��
��3������C��Ϊ�˳�ȥFe3+��Cu2+�����ʣ���ϡʱ����������ԭ���� ��
��4������C�м�ˮ����Ӧ������������������ˮ���Ժ���������ɵIJ���Ӱ���ǣ� ��
��5������E���еIJ����� ��
��6���Ƶõ��������к�����������ͭ��ͨ����ȥ����ͭ�ķ������ڲ���E֮ǰ���������Ƶ�Ag2O��ʹCu2+ת��ΪCu(OH)2��������Ӧ����˳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��14�֣�ÿ��2�֣�ij�Լ���������������ͭ�������ᣨ��Fe3+����Ӧ��ȡ���������������£�
��1����ҵ��һ��ѡ���е�Ũ�ȵ����������Ӧ����ȡ�������������±��ո���ա�
|
| �ŵ� | ȱ�� |
| ʹ��Ũ���� | ��Ӧ���ʿ� | ��Ľϴ���NOx�����϶� |
| ʹ��ϡ���� |
|
|
��2������B���ȱ��µ������� ��
a�� �����ڼӿ췴Ӧ����
b��������δ��Ӧ������ӷ�
c�������������ַ�Ӧ��������Һ��H+��Ũ��
��3������C��Ϊ�˳�ȥFe3+��Cu2+�����ʣ���ϡʱ����������ԭ���� ��
��4������C�м�ˮ����Ӧ������������������ˮ���Ժ���������ɵIJ���Ӱ���ǣ�
��
��5������E���еIJ����� ��
��6���Ƶõ��������к�����������ͭ��ͨ����ȥ����ͭ�ķ������ڲ���E֮ǰ���������Ƶ�Ag2O��ʹCu2+ת��ΪCu(OH)2��������Ӧ����˳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��