��Ŀ����
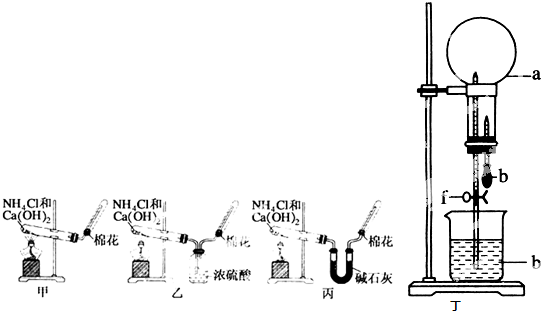
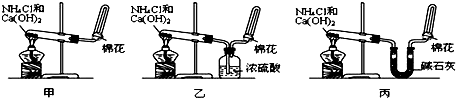
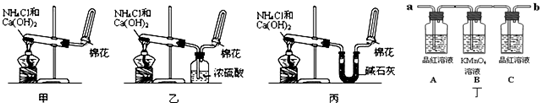
��14�֣��ס��ҡ�����λͬѧ�ֱ�����������ʵ��װ�ü���ѧҩƷ�����м�ʯ��Ϊ�����������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش��������⣺

��1����λͬѧ��ȡ�����Ļ�ѧ����ʽΪ��
_____________________________________________��
��2����λͬѧ���������ſ������ռ���������ԭ����____________________________.
��3����λͬѧ������װ����ȡ����ʱ,������һλͬѧû���ռ�������������ǵ�ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��___________��(���ס������ҡ�����)���ռ�������������Ҫԭ����_____________________________________(�û�ѧ����ʽ��ʾ)��
��4�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ��ۣ�_______________________
��5����λͬѧ����Ϊ���ǵ�ʵ��װ��Ҳ�����ڼ���̼����粒�����ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���_________(��ס������ҡ�����)����װ���е�NH4HCO3�����ܷ���NH4Cl��������Ʊ�NH3��____________(��ܡ����ܡ�)��
��14�֣�ÿ��2�֣�
��1��2NH4Cl+Ca��OH��2 CaCl2+2NH3��
+2H2O
CaCl2+2NH3��
+2H2O
��2��NH3���ܶ�С�ڿ���
��3���ң� 2NH3+H2SO4===��NH4��2SO4
��4����ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ�����ֽ��������˵�����ռ���������պ��Ũ����IJ����������Թܿڣ����а��̲�������֤�����ռ�����
��5������ ��
��������